+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Evernimicin bound to the 50S subunit | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Antibiotic / RIBOSOME | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationtranscriptional attenuation / endoribonuclease inhibitor activity / RNA-binding transcription regulator activity / positive regulation of ribosome biogenesis / negative regulation of cytoplasmic translation / DnaA-L2 complex / translation repressor activity / negative regulation of DNA-templated DNA replication initiation / mRNA regulatory element binding translation repressor activity / ribosome assembly ...transcriptional attenuation / endoribonuclease inhibitor activity / RNA-binding transcription regulator activity / positive regulation of ribosome biogenesis / negative regulation of cytoplasmic translation / DnaA-L2 complex / translation repressor activity / negative regulation of DNA-templated DNA replication initiation / mRNA regulatory element binding translation repressor activity / ribosome assembly / assembly of large subunit precursor of preribosome / cytosolic ribosome assembly / response to reactive oxygen species / regulation of cell growth / DNA-templated transcription termination / response to radiation / mRNA 5'-UTR binding / large ribosomal subunit / ribosome binding / 5S rRNA binding / large ribosomal subunit rRNA binding / transferase activity / ribosomal large subunit assembly / cytoplasmic translation / cytosolic large ribosomal subunit / tRNA binding / negative regulation of translation / rRNA binding / ribosome / structural constituent of ribosome / translation / response to antibiotic / negative regulation of DNA-templated transcription / mRNA binding / DNA binding / RNA binding / zinc ion binding / cytosol / cytoplasm Similarity search - Function | ||||||||||||
| Biological species |  | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 1.86 Å | ||||||||||||
 Authors Authors | Paternoga H / Crowe-McAuliffe C / Novacek J / Wilson DN | ||||||||||||
| Funding support | European Union, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2023 Journal: Nat Struct Mol Biol / Year: 2023Title: Structural conservation of antibiotic interaction with ribosomes. Authors: Helge Paternoga / Caillan Crowe-McAuliffe / Lars V Bock / Timm O Koller / Martino Morici / Bertrand Beckert / Alexander G Myasnikov / Helmut Grubmüller / Jiří Nováček / Daniel N Wilson /    Abstract: The ribosome is a major target for clinically used antibiotics, but multidrug resistant pathogenic bacteria are making our current arsenal of antimicrobials obsolete. Here we present cryo-electron- ...The ribosome is a major target for clinically used antibiotics, but multidrug resistant pathogenic bacteria are making our current arsenal of antimicrobials obsolete. Here we present cryo-electron-microscopy structures of 17 distinct compounds from six different antibiotic classes bound to the bacterial ribosome at resolutions ranging from 1.6 to 2.2 Å. The improved resolution enables a precise description of antibiotic-ribosome interactions, encompassing solvent networks that mediate multiple additional interactions between the drugs and their target. Our results reveal a high structural conservation in the binding mode between antibiotics with the same scaffold, including ordered water molecules. Water molecules are visualized within the antibiotic binding sites that are preordered, become ordered in the presence of the drug and that are physically displaced on drug binding. Insight into RNA-ligand interactions will facilitate development of new antimicrobial agents, as well as other RNA-targeting therapies. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16530.map.gz emd_16530.map.gz | 47.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16530-v30.xml emd-16530-v30.xml emd-16530.xml emd-16530.xml | 52.7 KB 52.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_16530.png emd_16530.png | 117 KB | ||
| Filedesc metadata |  emd-16530.cif.gz emd-16530.cif.gz | 11 KB | ||
| Others |  emd_16530_additional_1.map.gz emd_16530_additional_1.map.gz emd_16530_half_map_1.map.gz emd_16530_half_map_1.map.gz emd_16530_half_map_2.map.gz emd_16530_half_map_2.map.gz | 408.7 MB 411.7 MB 411.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16530 http://ftp.pdbj.org/pub/emdb/structures/EMD-16530 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16530 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16530 | HTTPS FTP |
-Validation report
| Summary document |  emd_16530_validation.pdf.gz emd_16530_validation.pdf.gz | 834.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_16530_full_validation.pdf.gz emd_16530_full_validation.pdf.gz | 834.3 KB | Display | |
| Data in XML |  emd_16530_validation.xml.gz emd_16530_validation.xml.gz | 18.5 KB | Display | |
| Data in CIF |  emd_16530_validation.cif.gz emd_16530_validation.cif.gz | 21.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16530 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16530 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16530 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16530 | HTTPS FTP |
-Related structure data
| Related structure data |  8camMC  8ca7C  8caiC  8cazC  8cepC  8ceuC  8cf1C  8cf8C  8cgdC  8cgiC  8cgjC  8cgkC  8cgrC  8cguC  8cgvC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_16530.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16530.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.762 Å | ||||||||||||||||||||||||||||||||||||
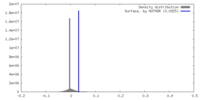
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: #1
| File | emd_16530_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
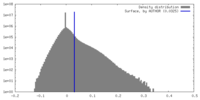
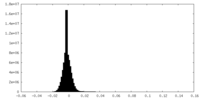
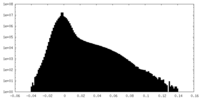
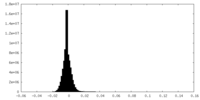
| Density Histograms |
-Half map: #2
| File | emd_16530_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
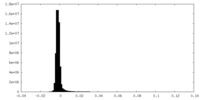
| Density Histograms |
-Half map: #1
| File | emd_16530_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
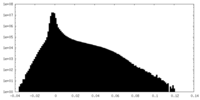
| Density Histograms |
- Sample components
Sample components
+Entire : 70S ribosomes with antibiotic cocktail
+Supramolecule #1: 70S ribosomes with antibiotic cocktail
+Macromolecule #1: Large ribosomal subunit protein bL33
+Macromolecule #2: Large ribosomal subunit protein bL34
+Macromolecule #3: Large ribosomal subunit protein bL35
+Macromolecule #4: Large ribosomal subunit protein bL36A
+Macromolecule #7: Large ribosomal subunit protein uL2
+Macromolecule #8: Large ribosomal subunit protein uL3
+Macromolecule #9: Large ribosomal subunit protein uL4
+Macromolecule #10: Large ribosomal subunit protein uL6
+Macromolecule #11: Large ribosomal subunit protein bL9
+Macromolecule #12: Large ribosomal subunit protein uL13
+Macromolecule #13: Large ribosomal subunit protein uL14
+Macromolecule #14: 50S ribosomal protein L15
+Macromolecule #15: Large ribosomal subunit protein uL16
+Macromolecule #16: Large ribosomal subunit protein bL17
+Macromolecule #17: Large ribosomal subunit protein bL19
+Macromolecule #18: Large ribosomal subunit protein bL20
+Macromolecule #19: Large ribosomal subunit protein bL21
+Macromolecule #20: Large ribosomal subunit protein uL22
+Macromolecule #21: Large ribosomal subunit protein uL23
+Macromolecule #22: Large ribosomal subunit protein uL24
+Macromolecule #23: 50S ribosomal protein L25
+Macromolecule #24: Large ribosomal subunit protein bL27
+Macromolecule #25: Large ribosomal subunit protein bL28
+Macromolecule #26: Large ribosomal subunit protein uL29
+Macromolecule #27: Large ribosomal subunit protein uL30
+Macromolecule #28: Large ribosomal subunit protein bL32
+Macromolecule #5: 23S rRNA
+Macromolecule #6: 5S rRNA
+Macromolecule #29: ZINC ION
+Macromolecule #30: ACETATE ION
+Macromolecule #31: MAGNESIUM ION
+Macromolecule #32: POTASSIUM ION
+Macromolecule #33: Evernimicin
+Macromolecule #34: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Component:
| |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GRAPHENE | |||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Number real images: 24195 / Average exposure time: 4.0 sec. / Average electron dose: 44.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.6 µm / Nominal defocus min: 0.4 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 1.86 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 514855 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller






















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)