+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | beta-2-microglobulin D76N amyloid fibril form 1PFa | ||||||||||||
 Map data Map data | CryoEM helical symmetrised map of b2m-D76N Form 1PFa (sharpening b-factor of -84) | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Amyloid / fibril / helical / cross-beta / dialysis-related amyloidosis / b2m / polymorph / PROTEIN FIBRIL | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of ferrous iron binding / positive regulation of transferrin receptor binding / positive regulation of receptor binding / early endosome lumen / Nef mediated downregulation of MHC class I complex cell surface expression / negative regulation of receptor binding / DAP12 interactions / cellular response to iron ion / Endosomal/Vacuolar pathway / Antigen Presentation: Folding, assembly and peptide loading of class I MHC ...positive regulation of ferrous iron binding / positive regulation of transferrin receptor binding / positive regulation of receptor binding / early endosome lumen / Nef mediated downregulation of MHC class I complex cell surface expression / negative regulation of receptor binding / DAP12 interactions / cellular response to iron ion / Endosomal/Vacuolar pathway / Antigen Presentation: Folding, assembly and peptide loading of class I MHC / cellular response to iron(III) ion / antigen processing and presentation of exogenous protein antigen via MHC class Ib, TAP-dependent / negative regulation of forebrain neuron differentiation / regulation of erythrocyte differentiation / peptide antigen assembly with MHC class I protein complex / ER to Golgi transport vesicle membrane / regulation of iron ion transport / response to molecule of bacterial origin / MHC class I peptide loading complex / HFE-transferrin receptor complex / T cell mediated cytotoxicity / positive regulation of T cell cytokine production / antigen processing and presentation of endogenous peptide antigen via MHC class I / MHC class I protein complex / negative regulation of neurogenesis / positive regulation of receptor-mediated endocytosis / peptide antigen assembly with MHC class II protein complex / multicellular organismal-level iron ion homeostasis / MHC class II protein complex / cellular response to nicotine / specific granule lumen / positive regulation of cellular senescence / positive regulation of T cell mediated cytotoxicity / recycling endosome membrane / phagocytic vesicle membrane / peptide antigen binding / antigen processing and presentation of exogenous peptide antigen via MHC class II / negative regulation of epithelial cell proliferation / Immunoregulatory interactions between a Lymphoid and a non-Lymphoid cell / positive regulation of immune response / Interferon gamma signaling / Modulation by Mtb of host immune system / positive regulation of T cell activation / sensory perception of smell / negative regulation of neuron projection development / positive regulation of protein binding / tertiary granule lumen / DAP12 signaling / MHC class II protein complex binding / late endosome membrane / iron ion transport / ER-Phagosome pathway / T cell differentiation in thymus / early endosome membrane / protein refolding / protein homotetramerization / intracellular iron ion homeostasis / amyloid fibril formation / learning or memory / Amyloid fiber formation / endoplasmic reticulum lumen / lysosomal membrane / Golgi membrane / external side of plasma membrane / focal adhesion / Neutrophil degranulation / SARS-CoV-2 activates/modulates innate and adaptive immune responses / structural molecule activity / Golgi apparatus / endoplasmic reticulum / protein homodimerization activity / extracellular space / extracellular exosome / extracellular region / identical protein binding / membrane / plasma membrane / cytosol Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
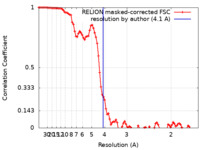
| Method | helical reconstruction / cryo EM / Resolution: 4.1 Å | ||||||||||||
 Authors Authors | Wilkinson M / Gallardo R / Guthertz N / Martinez RM / Radford SE / Ranson NA | ||||||||||||
| Funding support |  United Kingdom, 3 items United Kingdom, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Disease-relevant β-microglobulin variants share a common amyloid fold. Authors: Martin Wilkinson / Rodrigo U Gallardo / Roberto Maya Martinez / Nicolas Guthertz / Masatomo So / Liam D Aubrey / Sheena E Radford / Neil A Ranson /    Abstract: β-microglobulin (βm) and its truncated variant ΔΝ6 are co-deposited in amyloid fibrils in the joints, causing the disorder dialysis-related amyloidosis (DRA). Point mutations of βm result in ...β-microglobulin (βm) and its truncated variant ΔΝ6 are co-deposited in amyloid fibrils in the joints, causing the disorder dialysis-related amyloidosis (DRA). Point mutations of βm result in diseases with distinct pathologies. βm-D76N causes a rare systemic amyloidosis with protein deposited in the viscera in the absence of renal failure, whilst βm-V27M is associated with renal failure, with amyloid deposits forming predominantly in the tongue. Here we use cryoEM to determine the structures of fibrils formed from these variants under identical conditions in vitro. We show that each fibril sample is polymorphic, with diversity arising from a 'lego-like' assembly of a common amyloid building block. These results suggest a 'many sequences, one amyloid fold' paradigm in contrast with the recently reported 'one sequence, many amyloid folds' behaviour of intrinsically disordered proteins such as tau and Aβ. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15226.map.gz emd_15226.map.gz | 7.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15226-v30.xml emd-15226-v30.xml emd-15226.xml emd-15226.xml | 18.9 KB 18.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15226_fsc.xml emd_15226_fsc.xml | 9.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_15226.png emd_15226.png | 70.8 KB | ||
| Filedesc metadata |  emd-15226.cif.gz emd-15226.cif.gz | 5.6 KB | ||
| Others |  emd_15226_half_map_1.map.gz emd_15226_half_map_1.map.gz emd_15226_half_map_2.map.gz emd_15226_half_map_2.map.gz | 59.4 MB 59.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15226 http://ftp.pdbj.org/pub/emdb/structures/EMD-15226 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15226 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15226 | HTTPS FTP |
-Validation report
| Summary document |  emd_15226_validation.pdf.gz emd_15226_validation.pdf.gz | 936.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_15226_full_validation.pdf.gz emd_15226_full_validation.pdf.gz | 935.6 KB | Display | |
| Data in XML |  emd_15226_validation.xml.gz emd_15226_validation.xml.gz | 15.5 KB | Display | |
| Data in CIF |  emd_15226_validation.cif.gz emd_15226_validation.cif.gz | 21 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15226 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15226 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15226 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15226 | HTTPS FTP |
-Related structure data
| Related structure data |  8a7oC  8a7pC  8a7qC  8a7tC C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_15226.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15226.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CryoEM helical symmetrised map of b2m-D76N Form 1PFa (sharpening b-factor of -84) | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||||||||||||||||||
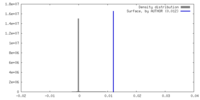
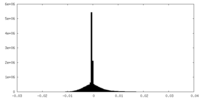
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: halfmap2
| File | emd_15226_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | halfmap2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
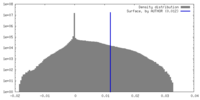
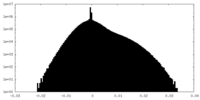
| Density Histograms |
-Half map: halfmap1
| File | emd_15226_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | halfmap1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Amyloid fibril polymorph 1PFa of the beta-2-microglobulin D76N va...
| Entire | Name: Amyloid fibril polymorph 1PFa of the beta-2-microglobulin D76N variant. |
|---|---|
| Components |
|
-Supramolecule #1: Amyloid fibril polymorph 1PFa of the beta-2-microglobulin D76N va...
| Supramolecule | Name: Amyloid fibril polymorph 1PFa of the beta-2-microglobulin D76N variant. type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: Recombinantly expressed and fibrillated in vitro at pH 6.2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: beta-2-microglobulin variant D76N
| Macromolecule | Name: beta-2-microglobulin variant D76N / type: protein_or_peptide / ID: 1 / Details: Natural b2m variant D76N / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Sequence | String: IQRTPKIQVY SRHPAENGKS NFLNCYVSGF HPSDIEVDLL KNGERIEKVE HSDLSFSKDW SFYLLYYTEF TPTEKNEYAC RVNHVTLSQP KIVKWDRDM UniProtKB: Beta-2-microglobulin |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 6.2 Component:
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: EMS Lacey Carbon / Material: COPPER / Mesh: 300 / Support film - Material: GRAPHENE OXIDE / Support film - topology: CONTINUOUS / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 60 sec. Details: The grid was plasma cleaned prior to 2x application of graphene oxide-DDM mixture, then grid was used immediately for sample application and vitrification | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: 6s blot. | |||||||||
| Details | Fibrillation conditions: 40 uM monomeric b2m-D76N at 37C with shaking at 600 rpm for 2-3 weeks |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Number grids imaged: 1 / Number real images: 3849 / Average exposure time: 6.2 sec. / Average electron dose: 43.0 e/Å2 Details: 1477 raw EER frames were collected per image and combined into 54 fractions for processing |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 96000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)