[English] 日本語
 Yorodumi
Yorodumi- EMDB-15027: Cryo-EM structure of catalytically active Spinacia oleracea cytoc... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
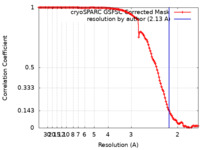
| Title | Cryo-EM structure of catalytically active Spinacia oleracea cytochrome b6f in complex with endogenous plastoquinones at 2.13 A resolution | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | cytochrome / Spinacia oleracea / plastoquinones / cryo-EM / PHOTOSYNTHESIS | |||||||||
| Function / homology |  Function and homology information Function and homology informationPSII associated light-harvesting complex II binding / chloroplast photosystem I binding / chloroplast photosystem II binding / cytochrome b6f complex / plastoquinol-plastocyanin reductase / plastoquinol--plastocyanin reductase activity / : / thylakoid membrane / cytochrome complex assembly / photosynthetic electron transport chain ...PSII associated light-harvesting complex II binding / chloroplast photosystem I binding / chloroplast photosystem II binding / cytochrome b6f complex / plastoquinol-plastocyanin reductase / plastoquinol--plastocyanin reductase activity / : / thylakoid membrane / cytochrome complex assembly / photosynthetic electron transport chain / : / chloroplast thylakoid membrane / response to light stimulus / photosynthesis / chloroplast / respiratory electron transport chain / 2 iron, 2 sulfur cluster binding / oxidoreductase activity / electron transfer activity / iron ion binding / heme binding / lipid binding / metal ion binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Spinacia oleracea (spinach) Spinacia oleracea (spinach) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.13 Å | |||||||||
 Authors Authors | Sarewicz M / Szwalec M / Pintscher S / Indyka P / Rawski M / Pietras R / Mielecki B / Koziej L / Jaciuk M / Glatt S / Osyczka A | |||||||||
| Funding support |  Poland, 2 items Poland, 2 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2023 Journal: Sci Adv / Year: 2023Title: High-resolution cryo-EM structures of plant cytochrome bf at work. Authors: Marcin Sarewicz / Mateusz Szwalec / Sebastian Pintscher / Paulina Indyka / Michał Rawski / Rafał Pietras / Bohun Mielecki / Łukasz Koziej / Marcin Jaciuk / Sebastian Glatt / Artur Osyczka /  Abstract: Plants use solar energy to power cellular metabolism. The oxidation of plastoquinol and reduction of plastocyanin by cytochrome bf (Cyt bf) is known as one of the key steps of photosynthesis, but the ...Plants use solar energy to power cellular metabolism. The oxidation of plastoquinol and reduction of plastocyanin by cytochrome bf (Cyt bf) is known as one of the key steps of photosynthesis, but the catalytic mechanism in the plastoquinone oxidation site (Q) remains elusive. Here, we describe two high-resolution cryo-EM structures of the spinach Cyt bf homodimer with endogenous plastoquinones and in complex with plastocyanin. Three plastoquinones are visible and line up one after another head to tail near Q in both monomers, indicating the existence of a channel in each monomer. Therefore, quinones appear to flow through Cyt bf in one direction, transiently exposing the redox-active ring of quinone during catalysis. Our work proposes an unprecedented one-way traffic model that explains efficient quinol oxidation during photosynthesis and respiration. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15027.map.gz emd_15027.map.gz | 98.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15027-v30.xml emd-15027-v30.xml emd-15027.xml emd-15027.xml | 39.3 KB 39.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15027_fsc.xml emd_15027_fsc.xml | 12.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_15027.png emd_15027.png | 129.5 KB | ||
| Filedesc metadata |  emd-15027.cif.gz emd-15027.cif.gz | 8.9 KB | ||
| Others |  emd_15027_additional_1.map.gz emd_15027_additional_1.map.gz emd_15027_half_map_1.map.gz emd_15027_half_map_1.map.gz emd_15027_half_map_2.map.gz emd_15027_half_map_2.map.gz | 185.4 MB 182.4 MB 182.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15027 http://ftp.pdbj.org/pub/emdb/structures/EMD-15027 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15027 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15027 | HTTPS FTP |
-Related structure data
| Related structure data |  7zyvMC  7qrmC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_15027.map.gz / Format: CCP4 / Size: 196.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15027.map.gz / Format: CCP4 / Size: 196.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.86 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: sharpened map
| File | emd_15027_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | sharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_15027_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_15027_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Cytochrome b6f complex
+Supramolecule #1: Cytochrome b6f complex
+Macromolecule #1: Cytochrome b6
+Macromolecule #2: Cytochrome b6-f complex subunit 4
+Macromolecule #3: Cytochrome f
+Macromolecule #4: Cytochrome b6-f complex iron-sulfur subunit, chloroplastic
+Macromolecule #5: Cytochrome b6-f complex subunit 6
+Macromolecule #6: Cytochrome b6-f complex subunit 7
+Macromolecule #7: Cytochrome b6-f complex subunit 5
+Macromolecule #8: Cytochrome b6-f complex subunit 8
+Macromolecule #9: Thylakoid soluble phosphoprotein
+Macromolecule #10: PROTOPORPHYRIN IX CONTAINING FE
+Macromolecule #11: HEME C
+Macromolecule #12: CHLOROPHYLL A
+Macromolecule #13: UNDECYL-MALTOSIDE
+Macromolecule #14: 2,3-DIMETHYL-5-(3,7,11,15,19,23,27,31,35-NONAMETHYL-2,6,10,14,18,...
+Macromolecule #15: 1,2-DI-O-ACYL-3-O-[6-DEOXY-6-SULFO-ALPHA-D-GLUCOPYRANOSYL]-SN-GLYCEROL
+Macromolecule #16: FE2/S2 (INORGANIC) CLUSTER
+Macromolecule #17: BETA-CAROTENE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 6.2 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
Details: Buffer and the diluted protein sample were filtered through a 0.22 um syringe filter. Prior to freezing, protein sample was concentrated on centrifugal filter units with 50K MWCO. | ||||||||||||
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 30.0 kPa | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: blot time 2 s, wait time 0 s, blot force -1. |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4092 pixel / Number real images: 7651 / Average exposure time: 1.82 sec. / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.1 µm / Nominal defocus min: 0.9 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)