+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Twist-corrected RNA origami 5-helix Tile A | |||||||||
 Map data Map data | Sharpened map with b factor of -252. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | RNA / Origami / nanostructure | |||||||||
| Biological species | synthetic construct (others) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 5.14 Å | |||||||||
 Authors Authors | McRae EKS / Bogglid A / Boesen T / Andersen ES | |||||||||
| Funding support |  Denmark, 1 items Denmark, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Nanotechnol / Year: 2023 Journal: Nat Nanotechnol / Year: 2023Title: Structure, folding and flexibility of co-transcriptional RNA origami. Authors: Ewan K S McRae / Helena Østergaard Rasmussen / Jianfang Liu / Andreas Bøggild / Michael T A Nguyen / Nestor Sampedro Vallina / Thomas Boesen / Jan Skov Pedersen / Gang Ren / Cody Geary / ...Authors: Ewan K S McRae / Helena Østergaard Rasmussen / Jianfang Liu / Andreas Bøggild / Michael T A Nguyen / Nestor Sampedro Vallina / Thomas Boesen / Jan Skov Pedersen / Gang Ren / Cody Geary / Ebbe Sloth Andersen /   Abstract: RNA origami is a method for designing RNA nanostructures that can self-assemble through co-transcriptional folding with applications in nanomedicine and synthetic biology. However, to advance the ...RNA origami is a method for designing RNA nanostructures that can self-assemble through co-transcriptional folding with applications in nanomedicine and synthetic biology. However, to advance the method further, an improved understanding of RNA structural properties and folding principles is required. Here we use cryogenic electron microscopy to study RNA origami sheets and bundles at sub-nanometre resolution revealing structural parameters of kissing-loop and crossover motifs, which are used to improve designs. In RNA bundle designs, we discover a kinetic folding trap that forms during folding and is only released after 10 h. Exploration of the conformational landscape of several RNA designs reveal the flexibility of helices and structural motifs. Finally, sheets and bundles are combined to construct a multidomain satellite shape, which is characterized by individual-particle cryo-electron tomography to reveal the domain flexibility. Together, the study provides a structural basis for future improvements to the design cycle of genetically encoded RNA nanodevices. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13926.map.gz emd_13926.map.gz | 220.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13926-v30.xml emd-13926-v30.xml emd-13926.xml emd-13926.xml | 19.1 KB 19.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_13926_fsc.xml emd_13926_fsc.xml | 17.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_13926.png emd_13926.png | 40.8 KB | ||
| Masks |  emd_13926_msk_1.map emd_13926_msk_1.map | 233.3 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-13926.cif.gz emd-13926.cif.gz | 5.8 KB | ||
| Others |  emd_13926_half_map_1.map.gz emd_13926_half_map_1.map.gz emd_13926_half_map_2.map.gz emd_13926_half_map_2.map.gz | 216.8 MB 216.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13926 http://ftp.pdbj.org/pub/emdb/structures/EMD-13926 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13926 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13926 | HTTPS FTP |
-Validation report
| Summary document |  emd_13926_validation.pdf.gz emd_13926_validation.pdf.gz | 958.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_13926_full_validation.pdf.gz emd_13926_full_validation.pdf.gz | 957.6 KB | Display | |
| Data in XML |  emd_13926_validation.xml.gz emd_13926_validation.xml.gz | 21.4 KB | Display | |
| Data in CIF |  emd_13926_validation.cif.gz emd_13926_validation.cif.gz | 27.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13926 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13926 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13926 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13926 | HTTPS FTP |
-Related structure data
| Related structure data |  7qduMC  7ptkC  7ptlC  7ptqC  7ptsC M: atomic model generated by this map C: citing same article ( |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_13926.map.gz / Format: CCP4 / Size: 233.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13926.map.gz / Format: CCP4 / Size: 233.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map with b factor of -252. | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.294 Å | ||||||||||||||||||||||||||||||||||||
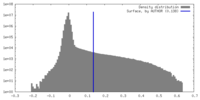
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_13926_msk_1.map emd_13926_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
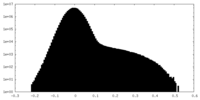
| Density Histograms |
-Half map: Half map B
| File | emd_13926_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
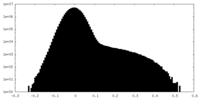
| Density Histograms |
-Half map: Half map A
| File | emd_13926_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Twist-corrected 5-helix tile A.
| Entire | Name: Twist-corrected 5-helix tile A. |
|---|---|
| Components |
|
-Supramolecule #1: Twist-corrected 5-helix tile A.
| Supramolecule | Name: Twist-corrected 5-helix tile A. / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: In vitro transcribed RNA purified by SEC. |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 175 KDa |
-Macromolecule #1: Chains: Q
| Macromolecule | Name: Chains: Q / type: rna / ID: 1 / Details: In vitro transcribed RNA / Number of copies: 1 |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 177.720031 KDa |
| Sequence | String: GGGCACUUAC CCUUUAGUGC GAAGGUUUCG ACCUUCGAUC CAUUUGUUCG CAAAUGGGAU GAUCUUCGGA UCAUCCGCGU AGUCUGUUC AGUCGUUUCG ACGACUGGCC CCACUUCGGU GGGGCCACGG UACUUAGAAG UGAACACUAA GUGUCGUGAA C ACCAUUUG ...String: GGGCACUUAC CCUUUAGUGC GAAGGUUUCG ACCUUCGAUC CAUUUGUUCG CAAAUGGGAU GAUCUUCGGA UCAUCCGCGU AGUCUGUUC AGUCGUUUCG ACGACUGGCC CCACUUCGGU GGGGCCACGG UACUUAGAAG UGAACACUAA GUGUCGUGAA C ACCAUUUG GUUAACUGCU CAAACUAAAU GGUGAUGAGG GAAGGAAUGA CCCUCAUCGG ACUACGCGAU CCGAGUGAUG GG AAUGGCU GACCCAUCGC UCGGCACUGG AGGGUGAGUG CCCCUCAUUC GCAUAAGGGC CGACCCAGAC AACAGCCAAG UUU GGGUCG GAGAUGCGAA CAUUCCACGC AUCUGAACGG UUGAGAACUU ACAAGGGCAA GAGCAGAGUC CUUGUAAGGG CUUU ACACG UCAAGUUCAC AGACGUGUAA GGCCCGUCGC CCUUCGGGGC GACGUUCACG GCAUUUCGAU GCCGUGCAGC CUGUU CGCA GGCUGCUUGA CCGUUCCCCU GCCCUUUCGA GGGCAGACUA CUCUUCGGAG UAGUCUUAUG UGAAUGAG |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3.0 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
Details: Freshly prepared and filtered through 0.22 micron filter prior to use. | ||||||||||||
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 45 sec. / Pretreatment - Atmosphere: AIR / Details: 15mA current on a glocube. | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 99 % / Chamber temperature: 294 K / Instrument: LEICA EM GP Details: 3 microlitre droplet, 4 second delay before blotting, 6 second blot, 0 second delay before plunging.. | ||||||||||||
| Details | Sample was purified by size exclusion chromatography and concentrated in an Amicon spin concentrator. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 6122 / Average exposure time: 1.5 sec. / Average electron dose: 60.0 e/Å2 / Details: Images were collected as 56 frame movies. |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Target criteria: Correlation Coefficient |
|---|---|
| Output model |  PDB-7qdu: |
 Movie
Movie Controller
Controller



























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)