[English] 日本語
 Yorodumi
Yorodumi- EMDB-13504: Cryo-EM structure of the actomyosin-V complex in the rigor state ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-13504 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the actomyosin-V complex in the rigor state (central 1er, class 2) | ||||||||||||||||||
 Map data Map data | Sharpened map of the central actomyosin-V-LC molecule filtered to local resolution (class 2) | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationminus-end directed microfilament motor activity / unconventional myosin complex / insulin-responsive compartment / muscle myosin complex / muscle filament sliding / myosin complex / myosin II complex / structural constituent of muscle / cytoskeletal motor activator activity / myosin heavy chain binding ...minus-end directed microfilament motor activity / unconventional myosin complex / insulin-responsive compartment / muscle myosin complex / muscle filament sliding / myosin complex / myosin II complex / structural constituent of muscle / cytoskeletal motor activator activity / myosin heavy chain binding / microfilament motor activity / tropomyosin binding / actin filament bundle / troponin I binding / filamentous actin / mesenchyme migration / cytoskeletal motor activity / skeletal muscle myofibril / actin filament bundle assembly / striated muscle thin filament / skeletal muscle thin filament assembly / actin monomer binding / Smooth Muscle Contraction / skeletal muscle tissue development / skeletal muscle fiber development / stress fiber / vesicle-mediated transport / titin binding / actin filament polymerization / muscle contraction / actin filament organization / protein localization to plasma membrane / actin filament / filopodium / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / cellular response to insulin stimulus / calcium-dependent protein binding / actin filament binding / actin cytoskeleton / lamellipodium / cell body / calmodulin binding / Golgi membrane / protein domain specific binding / hydrolase activity / calcium ion binding / positive regulation of gene expression / magnesium ion binding / ATP hydrolysis activity / extracellular exosome / ATP binding / identical protein binding / membrane / cytoplasm / cytosol Similarity search - Function | ||||||||||||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /    | ||||||||||||||||||
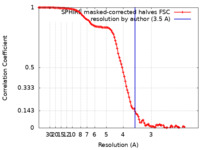
| Method | helical reconstruction / cryo EM / Resolution: 3.5 Å | ||||||||||||||||||
 Authors Authors | Pospich S / Sweeney HL / Houdusse A / Raunser S | ||||||||||||||||||
| Funding support |  Germany, European Union, Germany, European Union,  France, France,  United States, 5 items United States, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Elife / Year: 2021 Journal: Elife / Year: 2021Title: High-resolution structures of the actomyosin-V complex in three nucleotide states provide insights into the force generation mechanism. Authors: Sabrina Pospich / H Lee Sweeney / Anne Houdusse / Stefan Raunser /    Abstract: The molecular motor myosin undergoes a series of major structural transitions during its force-producing motor cycle. The underlying mechanism and its coupling to ATP hydrolysis and actin binding are ...The molecular motor myosin undergoes a series of major structural transitions during its force-producing motor cycle. The underlying mechanism and its coupling to ATP hydrolysis and actin binding are only partially understood, mostly due to sparse structural data on actin-bound states of myosin. Here, we report 26 high-resolution cryo-EM structures of the actomyosin-V complex in the strong-ADP, rigor, and a previously unseen post-rigor transition state that binds the ATP analog AppNHp. The structures reveal a high flexibility of myosin in each state and provide valuable insights into the structural transitions of myosin-V upon ADP release and binding of AppNHp, as well as the actomyosin interface. In addition, they show how myosin is able to specifically alter the structure of F-actin. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13504.map.gz emd_13504.map.gz | 1.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13504-v30.xml emd-13504-v30.xml emd-13504.xml emd-13504.xml | 29.3 KB 29.3 KB | Display Display |  EMDB header EMDB header |
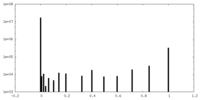
| FSC (resolution estimation) |  emd_13504_fsc.xml emd_13504_fsc.xml | 11.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_13504.png emd_13504.png | 96.6 KB | ||
| Masks |  emd_13504_msk_1.map emd_13504_msk_1.map | 125 MB |  Mask map Mask map | |
| Others |  emd_13504_additional_1.map.gz emd_13504_additional_1.map.gz emd_13504_additional_2.map.gz emd_13504_additional_2.map.gz emd_13504_half_map_1.map.gz emd_13504_half_map_1.map.gz emd_13504_half_map_2.map.gz emd_13504_half_map_2.map.gz | 1.5 MB 12.1 MB 59.5 MB 59.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13504 http://ftp.pdbj.org/pub/emdb/structures/EMD-13504 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13504 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13504 | HTTPS FTP |
-Related structure data
| Related structure data |  7plwMC  7pltC  7pluC  7plvC  7plxC  7plyC  7plzC  7pm0C  7pm1C  7pm2C  7pm3C  7pm5C  7pm6C  7pm7C  7pm8C  7pm9C  7pmaC  7pmbC  7pmcC  7pmdC  7pmeC  7pmfC  7pmgC  7pmhC  7pmiC  7pmjC  7pmlC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_13504.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13504.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map of the central actomyosin-V-LC molecule filtered to local resolution (class 2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
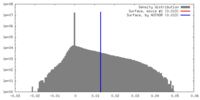
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_13504_msk_1.map emd_13504_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Sharpened map of the central actomyosin-V-LC molecule filtered...
| File | emd_13504_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map of the central actomyosin-V-LC molecule filtered to nominal resolution (class 2) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Denoised map of the central actomyosin-V-LC molecule (LAFTER,...
| File | emd_13504_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Denoised map of the central actomyosin-V-LC molecule (LAFTER, class 2) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map (signal subtracted particles, class 2)
| File | emd_13504_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map (signal subtracted particles, class 2) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map (signal subtracted particles, class 2)
| File | emd_13504_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map (signal subtracted particles, class 2) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Actomyosin-V complex in the rigor state
+Supramolecule #1: Actomyosin-V complex in the rigor state
+Supramolecule #2: Myosin light chain 6B
+Supramolecule #3: Unconventional myosin-Va
+Supramolecule #4: Actin, alpha skeletal muscle
+Supramolecule #5: Phalloidin
+Macromolecule #1: Myosin light chain 6B
+Macromolecule #2: Unconventional myosin-Va
+Macromolecule #3: Actin, alpha skeletal muscle
+Macromolecule #4: Phalloidin
+Macromolecule #5: ADENOSINE-5'-DIPHOSPHATE
+Macromolecule #6: MAGNESIUM ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 286 K / Instrument: FEI VITROBOT MARK III / Details: On grid decoration. |
| Details | Signal subtracted |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Number grids imaged: 1 / Number real images: 3623 / Average exposure time: 15.0 sec. / Average electron dose: 79.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller







































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)