[English] 日本語
 Yorodumi
Yorodumi- EMDB-0001: Cryo-EM structure of bacterial RNA polymerase-sigma54 holoenzyme ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of bacterial RNA polymerase-sigma54 holoenzyme transcription open complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | transcription initiation / DNA opening / transcription bubble / open complex / TRANSCRIPTION | |||||||||
| Function / homology |  Function and homology information Function and homology informationnitrogen fixation / DNA-binding transcription activator activity / RNA polymerase complex / submerged biofilm formation / cellular response to cell envelope stress / bacterial-type RNA polymerase core enzyme binding / cytosolic DNA-directed RNA polymerase complex / regulation of DNA-templated transcription initiation / sigma factor activity / bacterial-type flagellum assembly ...nitrogen fixation / DNA-binding transcription activator activity / RNA polymerase complex / submerged biofilm formation / cellular response to cell envelope stress / bacterial-type RNA polymerase core enzyme binding / cytosolic DNA-directed RNA polymerase complex / regulation of DNA-templated transcription initiation / sigma factor activity / bacterial-type flagellum assembly / bacterial-type flagellum-dependent cell motility / nitrate assimilation / DNA-directed RNA polymerase complex / nucleotidyltransferase activity / transcription elongation factor complex / regulation of DNA-templated transcription elongation / DNA-templated transcription initiation / transcription antitermination / cell motility / ribonucleoside binding / DNA-directed 5'-3' RNA polymerase activity / DNA-directed RNA polymerase / response to heat / protein-containing complex assembly / intracellular iron ion homeostasis / protein dimerization activity / response to antibiotic / magnesium ion binding / DNA binding / zinc ion binding / membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |   Klebsiella pneumoniae (bacteria) / Klebsiella pneumoniae (bacteria) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Glyde R / Ye FZ | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2018 Journal: Mol Cell / Year: 2018Title: Structures of Bacterial RNA Polymerase Complexes Reveal the Mechanism of DNA Loading and Transcription Initiation. Authors: Robert Glyde / Fuzhou Ye / Milija Jovanovic / Ioly Kotta-Loizou / Martin Buck / Xiaodong Zhang /  Abstract: Gene transcription is carried out by multi-subunit RNA polymerases (RNAPs). Transcription initiation is a dynamic multi-step process that involves the opening of the double-stranded DNA to form a ...Gene transcription is carried out by multi-subunit RNA polymerases (RNAPs). Transcription initiation is a dynamic multi-step process that involves the opening of the double-stranded DNA to form a transcription bubble and delivery of the template strand deep into the RNAP for RNA synthesis. Applying cryoelectron microscopy to a unique transcription system using σ (σ), the major bacterial variant sigma factor, we capture a new intermediate state at 4.1 Å where promoter DNA is caught at the entrance of the RNAP cleft. Combining with new structures of the open promoter complex and an initial de novo transcribing complex at 3.4 and 3.7 Å, respectively, our studies reveal the dynamics of DNA loading and mechanism of transcription bubble stabilization that involves coordinated, large-scale conformational changes of the universally conserved features within RNAP and DNA. In addition, our studies reveal a novel mechanism of strand separation by σ. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|---|
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0001.map.gz emd_0001.map.gz | 40 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0001-v30.xml emd-0001-v30.xml emd-0001.xml emd-0001.xml | 23.7 KB 23.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_0001.png emd_0001.png | 1.7 MB | ||
| Filedesc metadata |  emd-0001.cif.gz emd-0001.cif.gz | 8.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0001 http://ftp.pdbj.org/pub/emdb/structures/EMD-0001 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0001 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0001 | HTTPS FTP |
-Validation report
| Summary document |  emd_0001_validation.pdf.gz emd_0001_validation.pdf.gz | 234.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_0001_full_validation.pdf.gz emd_0001_full_validation.pdf.gz | 233.8 KB | Display | |
| Data in XML |  emd_0001_validation.xml.gz emd_0001_validation.xml.gz | 6.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0001 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0001 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0001 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0001 | HTTPS FTP |
-Related structure data
| Related structure data |  6gh5MC  0002C  4397C  6gfwC  6gh6C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_0001.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0001.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||
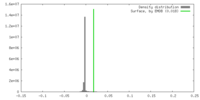
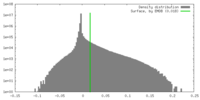
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
+Entire : Cryo-EM structure of bacterial RNA polymerase-sigma54 holoenzyme ...
+Supramolecule #1: Cryo-EM structure of bacterial RNA polymerase-sigma54 holoenzyme ...
+Supramolecule #2: DNA-directed RNA polymerase
+Supramolecule #3: RNA polymerase sigma-54 factor
+Supramolecule #4: DNA
+Macromolecule #1: DNA-directed RNA polymerase subunit alpha
+Macromolecule #2: DNA-directed RNA polymerase subunit beta
+Macromolecule #3: DNA-directed RNA polymerase subunit beta'
+Macromolecule #4: DNA-directed RNA polymerase subunit omega
+Macromolecule #5: RNA polymerase sigma-54 factor,RNA polymerase sigma-54 factor,RNA...
+Macromolecule #6: nifH promoter template DNA
+Macromolecule #7: nifH promoter non-template DNA
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 45.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 3.4 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 79678 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-6gh5: |
 Movie
Movie Controller
Controller


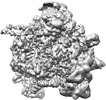













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)