+Search query
-Structure paper
| Title | A thermostable, closed SARS-CoV-2 spike protein trimer. |
|---|---|
| Journal, issue, pages | Nat Struct Mol Biol, Vol. 27, Issue 10, Page 934-941, Year 2020 |
| Publish date | Jul 31, 2020 |
 Authors Authors | Xiaoli Xiong / Kun Qu / Katarzyna A Ciazynska / Myra Hosmillo / Andrew P Carter / Soraya Ebrahimi / Zunlong Ke / Sjors H W Scheres / Laura Bergamaschi / Guinevere L Grice / Ying Zhang / / James A Nathan / Stephen Baker / Leo C James / Helen E Baxendale / Ian Goodfellow / Rainer Doffinger / John A G Briggs /   |
| PubMed Abstract | The spike (S) protein of SARS-CoV-2 mediates receptor binding and cell entry and is the dominant target of the immune system. It exhibits substantial conformational flexibility. It transitions from ...The spike (S) protein of SARS-CoV-2 mediates receptor binding and cell entry and is the dominant target of the immune system. It exhibits substantial conformational flexibility. It transitions from closed to open conformations to expose its receptor-binding site and, subsequently, from prefusion to postfusion conformations to mediate fusion of viral and cellular membranes. S-protein derivatives are components of vaccine candidates and diagnostic assays, as well as tools for research into the biology and immunology of SARS-CoV-2. Here we have designed mutations in S that allow the production of thermostable, disulfide-bonded S-protein trimers that are trapped in the closed, prefusion state. Structures of the disulfide-stabilized and non-disulfide-stabilized proteins reveal distinct closed and locked conformations of the S trimer. We demonstrate that the designed, thermostable, closed S trimer can be used in serological assays. This protein has potential applications as a reagent for serology, virology and as an immunogen. |
 External links External links |  Nat Struct Mol Biol / Nat Struct Mol Biol /  PubMed:32737467 / PubMed:32737467 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 3.0 - 3.5 Å |
| Structure data | EMDB-11329, PDB-6zox: EMDB-11330, PDB-6zoy: EMDB-11331, PDB-6zoz: EMDB-11332, PDB-6zp0: EMDB-11333, PDB-6zp1: EMDB-11334, PDB-6zp2: |
| Chemicals |  ChemComp-NAG: 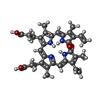 ChemComp-BLA:  ChemComp-EIC: |
| Source |
|
 Keywords Keywords | VIRAL PROTEIN / coronavirus / SARS-CoV-2 / Spike protein / S protein / S antigen / COVID-19 / receptor binding / membrane fusion / vaccine design |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers
















