+Search query
-Structure paper
| Title | Interaction of the tetratricopeptide repeat domain of aryl hydrocarbon receptor-interacting protein-like 1 with the regulatory Pγ subunit of phosphodiesterase 6. |
|---|---|
| Journal, issue, pages | J Biol Chem, Vol. 294, Issue 43, Page 15795-15807, Year 2019 |
| Publish date | Oct 25, 2019 |
 Authors Authors | Ravi P Yadav / Kimberly Boyd / Liping Yu / Nikolai O Artemyev /  |
| PubMed Abstract | Phosphodiesterase-6 (PDE6) is key to both phototransduction and health of rods and cones. Proper folding of PDE6 relies on the chaperone activity of aryl hydrocarbon receptor-interacting protein-like ...Phosphodiesterase-6 (PDE6) is key to both phototransduction and health of rods and cones. Proper folding of PDE6 relies on the chaperone activity of aryl hydrocarbon receptor-interacting protein-like 1 (AIPL1), and mutations in both PDE6 and AIPL1 can cause a severe form of blindness. Although AIPL1 and PDE6 are known to interact via the FK506-binding protein domain of AIPL1, the contribution of the tetratricopeptide repeat (TPR) domain of AIPL1 to its chaperone function is poorly understood. Here, we demonstrate that AIPL1-TPR interacts specifically with the regulatory Pγ subunit of PDE6. Use of NMR chemical shift perturbation (CSP) mapping technique revealed the interface between the C-terminal portion of Pγ and AIPL1-TPR. Our solution of the crystal structure of the AIPL1-TPR domain provided additional information, which together with the CSP data enabled us to generate a model of this interface. Biochemical analysis of chimeric AIPL1-AIP proteins supported this model and also revealed a correlation between the affinity of AIPL1-TPR for Pγ and the ability of Pγ to potentiate the chaperone activity of AIPL1. Based on these results, we present a model of the larger AIPL1-PDE6 complex. This supports the importance of simultaneous interactions of AIPL1-FK506-binding protein with the prenyl moieties of PDE6 and AIPL1-TPR with the Pγ subunit during the folding and/or assembly of PDE6. This study sheds new light on the versatility of TPR domains in protein folding by describing a novel TPR-protein binding partner, Pγ, and revealing that this subunit imparts AIPL1 selectivity for its client. |
 External links External links |  J Biol Chem / J Biol Chem /  PubMed:31488544 / PubMed:31488544 /  PubMed Central PubMed Central |
| Methods | SAS (X-ray synchrotron) / X-ray diffraction |
| Resolution | 1.55 Å |
| Structure data | 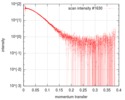 SASDGX4: Aryl-hydrocarbon-interacting protein-like 1 (Aryl-hydrocarbon-interacting protein-like 1(1-316), AIPL1)  PDB-6px0: |
| Chemicals |  ChemComp-PEG:  ChemComp-PG4:  ChemComp-BME:  ChemComp-EDO:  ChemComp-HOH: |
| Source |
|
 Keywords Keywords | ISOMERASE / AIPL1 TPR |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers



 homo sapiens (human)
homo sapiens (human)