+Search query
-Structure paper
| Title | Human cGAS catalytic domain has an additional DNA-binding interface that enhances enzymatic activity and liquid-phase condensation. |
|---|---|
| Journal, issue, pages | Proc. Natl. Acad. Sci. USA, Vol. 116, Page 11946-11955, Year 2019 |
| Publish date | Aug 9, 2018 (structure data deposition date) |
 Authors Authors | Xie, W. / Lama, L. / Adura, C. / Tomita, D. / Glickman, J.F. / Tuschl, T. / Patel, D.J. |
 External links External links |  Proc. Natl. Acad. Sci. USA / Proc. Natl. Acad. Sci. USA /  PubMed:31142647 PubMed:31142647 |
| Methods | X-ray diffraction |
| Resolution | 2.196 - 3.209 Å |
| Structure data |  PDB-6edb:  PDB-6edc:  PDB-6o47: |
| Chemicals |  ChemComp-ZN:  ChemComp-CIT:  ChemComp-LLS: 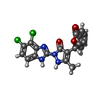 ChemComp-AEV:  ChemComp-HOH: |
| Source |
|
 Keywords Keywords | Transferase/DNA / SRY human cGAS DNA complex / Transferase-DNA complex / human cGAS DNA complex / TRANSFERASE / human / cGAS / core domain / compound / RU-521 / DNA BINDING PROTEIN |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers



 homo sapiens (human)
homo sapiens (human)