+Search query
-Structure paper
| Title | Structure of ThiM from Vitamin B1 biosynthetic pathway of Staphylococcus aureus - Insights into a novel pro-drug approach addressing MRSA infections. |
|---|---|
| Journal, issue, pages | Sci Rep, Vol. 6, Page 22871, Year 2016 |
| Publish date | Mar 10, 2016 |
 Authors Authors | Julia Drebes / Madeleine Künz / Björn Windshügel / Alexey G Kikhney / Ingrid B Müller / Raphael J Eberle / Dominik Oberthür / Huaixing Cang / Dmitri I Svergun / Markus Perbandt / Christian Betzel / Carsten Wrenger /    |
| PubMed Abstract | Infections caused by the methicillin-resistant Staphylococcus aureus (MRSA) are today known to be a substantial threat for global health. Emerging multi-drug resistant bacteria have created a ...Infections caused by the methicillin-resistant Staphylococcus aureus (MRSA) are today known to be a substantial threat for global health. Emerging multi-drug resistant bacteria have created a substantial need to identify and discover new drug targets and to develop novel strategies to treat bacterial infections. A promising and so far untapped antibiotic target is the biosynthesis of vitamin B1 (thiamin). Thiamin in its activated form, thiamin pyrophosphate, is an essential co-factor for all organisms. Therefore, thiamin analogous compounds, when introduced into the vitamin B1 biosynthetic pathway and further converted into non-functional co-factors by the bacterium can function as pro-drugs which thus block various co-factor dependent pathways. We characterized one of the key enzymes within the S. aureus vitamin B1 biosynthetic pathway, 5-(hydroxyethyl)-4-methylthiazole kinase (SaThiM; EC 2.7.1.50), a potential target for pro-drug compounds and analyzed the native structure of SaThiM and complexes with the natural substrate 5-(hydroxyethyl)-4-methylthiazole (THZ) and two selected substrate analogues. |
 External links External links |  Sci Rep / Sci Rep /  PubMed:26960569 / PubMed:26960569 /  PubMed Central PubMed Central |
| Methods | SAS (X-ray synchrotron) / X-ray diffraction |
| Resolution | 1.62 - 2.09 Å |
| Structure data |  SASDAX8:  PDB-5cga:  PDB-5cge:  PDB-5cm5:  PDB-5coj: |
| Chemicals | 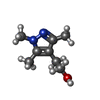 ChemComp-KP6:  ChemComp-MG:  ChemComp-HOH: 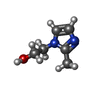 ChemComp-51F:  ChemComp-TZE: |
| Source |
|
 Keywords Keywords | TRANSFERASE / Bacterial Thiamine Biosynthesis / Hydroxyethylthiazole Kinase / Substrate Analog / 2-(4-methyl-1 / 3-thiazol-5-yl)ethanol |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers




