+Search query
-Structure paper
| Title | Structural studies of the giant mimivirus. |
|---|---|
| Journal, issue, pages | PLoS Biol, Vol. 7, Issue 4, Page e92, Year 2009 |
| Publish date | Apr 28, 2009 |
 Authors Authors | Chuan Xiao / Yurii G Kuznetsov / Siyang Sun / Susan L Hafenstein / Victor A Kostyuchenko / Paul R Chipman / Marie Suzan-Monti / Didier Raoult / Alexander McPherson / Michael G Rossmann /  |
| PubMed Abstract | Mimivirus is the largest known virus whose genome and physical size are comparable to some small bacteria, blurring the boundary between a virus and a cell. Structural studies of Mimivirus have been ...Mimivirus is the largest known virus whose genome and physical size are comparable to some small bacteria, blurring the boundary between a virus and a cell. Structural studies of Mimivirus have been difficult because of its size and long surface fibers. Here we report the use of enzymatic digestions to remove the surface fibers of Mimivirus in order to expose the surface of the viral capsid. Cryo-electron microscopy (cryoEM) and atomic force microscopy were able to show that the 20 icosahedral faces of Mimivirus capsids have hexagonal arrays of depressions. Each depression is surrounded by six trimeric capsomers that are similar in structure to those in many other large, icosahedral double-stranded DNA viruses. Whereas in most viruses these capsomers are hexagonally close-packed with the same orientation in each face, in Mimivirus there are vacancies at the systematic depressions with neighboring capsomers differing in orientation by 60 degrees . The previously observed starfish-shaped feature is well-resolved and found to be on each virus particle and is associated with a special pentameric vertex. The arms of the starfish fit into the gaps between the five faces surrounding the unique vertex, acting as a seal. Furthermore, the enveloped nucleocapsid is accurately positioned and oriented within the capsid with a concave surface facing the unique vertex. Thus, the starfish-shaped feature and the organization of the nucleocapsid might regulate the delivery of the genome to the host. The structure of Mimivirus, as well as the various fiber components observed in the virus, suggests that the Mimivirus genome includes genes derived from both eukaryotic and prokaryotic organisms. The three-dimensional cryoEM reconstruction reported here is of a virus with a volume that is one order of magnitude larger than any previously reported molecular assembly studied at a resolution of equal to or better than 65 Angstroms. |
 External links External links |  PLoS Biol / PLoS Biol /  PubMed:19402750 / PubMed:19402750 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 65.0 Å |
| Structure data | 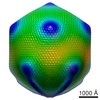 EMDB-5039: |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers



