+Search query
-Structure paper
| Title | Substrate transport and drug interaction of human thiamine transporters SLC19A2/A3. |
|---|---|
| Journal, issue, pages | Nat Commun, Vol. 15, Issue 1, Page 10924, Year 2024 |
| Publish date | Dec 30, 2024 |
 Authors Authors | Peipei Li / Zhini Zhu / Yong Wang / Xuyuan Zhang / Chuanhui Yang / Yalan Zhu / Zixuan Zhou / Yulin Chao / Yonghui Long / Yina Gao / Songqing Liu / Liguo Zhang / Pu Gao / Qianhui Qu /  |
| PubMed Abstract | Thiamine and pyridoxine are essential B vitamins that serve as enzymatic cofactors in energy metabolism, protein and nucleic acid biosynthesis, and neurotransmitter production. In humans, thiamine ...Thiamine and pyridoxine are essential B vitamins that serve as enzymatic cofactors in energy metabolism, protein and nucleic acid biosynthesis, and neurotransmitter production. In humans, thiamine transporters SLC19A2 and SLC19A3 primarily regulate cellular uptake of both vitamins. Genetic mutations in these transporters, which cause thiamine and pyridoxine deficiency, have been implicated in severe neurometabolic diseases. Additionally, various prescribed medicines, including metformin and fedratinib, manipulate thiamine transporters, complicating the therapeutic effect. Despite their physiological and pharmacological significance, the molecular underpinnings of substrate and drug recognition remain unknown. Here we present ten cryo-EM structures of human thiamine transporters SLC19A3 and SLC19A2 in outward- and inward-facing conformations, complexed with thiamine, pyridoxine, metformin, fedratinib, and amprolium. These structural insights, combined with functional characterizations, illuminate the translocation mechanism of diverse chemical entities, and enhance our understanding of drug-nutrient interactions mediated by thiamine transporters. |
 External links External links |  Nat Commun / Nat Commun /  PubMed:39738067 / PubMed:39738067 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 3.02 - 3.7 Å |
| Structure data | EMDB-39825, PDB-8z7r: EMDB-39826, PDB-8z7s: EMDB-39827, PDB-8z7t: EMDB-39828, PDB-8z7u: EMDB-39829, PDB-8z7v: EMDB-39830, PDB-8z7w: EMDB-39831, PDB-8z7x: EMDB-39832, PDB-8z7y: EMDB-39833, PDB-8z7z: EMDB-39834, PDB-8z80: |
| Chemicals |  ChemComp-VIB: 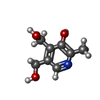 ChemComp-UEG: 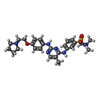 ChemComp-2TA:  PDB-1h5c:  ChemComp-MF8: |
| Source |
|
 Keywords Keywords | MEMBRANE PROTEIN / Thiamine transporter / MFS |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers























 homo sapiens (human)
homo sapiens (human)