+Search query
-Structure paper
| Title | Structures of full-length glycoprotein hormone receptor signalling complexes. |
|---|---|
| Journal, issue, pages | Nature, Vol. 598, Issue 7882, Page 688-692, Year 2021 |
| Publish date | Sep 22, 2021 |
 Authors Authors | Jia Duan / Peiyu Xu / Xi Cheng / Chunyou Mao / Tristan Croll / Xinheng He / Jingjing Shi / Xiaodong Luan / Wanchao Yin / Erli You / Qiufeng Liu / Shuyang Zhang / Hualiang Jiang / Yan Zhang / Yi Jiang / H Eric Xu /   |
| PubMed Abstract | Luteinizing hormone and chorionic gonadotropin are glycoprotein hormones that are related to follicle-stimulating hormone and thyroid-stimulating hormone. Luteinizing hormone and chorionic ...Luteinizing hormone and chorionic gonadotropin are glycoprotein hormones that are related to follicle-stimulating hormone and thyroid-stimulating hormone. Luteinizing hormone and chorionic gonadotropin are essential to human reproduction and are important therapeutic drugs. They activate the same G-protein-coupled receptor, luteinizing hormone-choriogonadotropin receptor (LHCGR), by binding to the large extracellular domain. Here we report four cryo-electron microscopy structures of LHCGR: two structures of the wild-type receptor in the inactive and active states; and two structures of the constitutively active mutated receptor. The active structures are bound to chorionic gonadotropin and the stimulatory G protein (G), and one of the structures also contains Org43553, an allosteric agonist. The structures reveal a distinct 'push-and-pull' mechanism of receptor activation, in which the extracellular domain is pushed by the bound hormone and pulled by the extended hinge loop next to the transmembrane domain. A highly conserved 10-residue fragment (P10) from the hinge C-terminal loop at the interface between the extracellular domain and the transmembrane domain functions as a tethered agonist to induce conformational changes in the transmembrane domain and G-protein coupling. Org43553 binds to a pocket of the transmembrane domain and interacts directly with P10, which further stabilizes the active conformation. Together, these structures provide a common model for understanding the signalling of glycoprotein hormone receptors and a basis for drug discovery for endocrine diseases. |
 External links External links |  Nature / Nature /  PubMed:34552239 PubMed:34552239 |
| Methods | EM (single particle) |
| Resolution | 3.2 - 4.3 Å |
| Structure data | EMDB-31596, PDB-7fig: EMDB-31597, PDB-7fih: EMDB-31598, PDB-7fii: EMDB-31599, PDB-7fij: |
| Chemicals |  ChemComp-NAG: 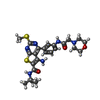 ChemComp-55Z: |
| Source |
|
 Keywords Keywords | MEMBRANE PROTEIN / glycoprotein hormone receptor / luteinizing hormone / chorionic gonadotropin / GPCR / Gs-protein / choriogonadotropin receptor |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers











 homo sapiens (human)
homo sapiens (human)
