+Search query
-Structure paper
| Title | Small molecule inhibitors of 15-PGDH exploit a physiologic induced-fit closing system. |
|---|---|
| Journal, issue, pages | Nat Commun, Vol. 14, Issue 1, Page 784, Year 2023 |
| Publish date | Feb 11, 2023 |
 Authors Authors | Wei Huang / Hongyun Li / Janna Kiselar / Stephen P Fink / Sagar Regmi / Alexander Day / Yiyuan Yuan / Mark Chance / Joseph M Ready / Sanford D Markowitz / Derek J Taylor /  |
| PubMed Abstract | 15-prostaglandin dehydrogenase (15-PGDH) is a negative regulator of tissue stem cells that acts via enzymatic activity of oxidizing and degrading PGE2, and related eicosanoids, that support stem ...15-prostaglandin dehydrogenase (15-PGDH) is a negative regulator of tissue stem cells that acts via enzymatic activity of oxidizing and degrading PGE2, and related eicosanoids, that support stem cells during tissue repair. Indeed, inhibiting 15-PGDH markedly accelerates tissue repair in multiple organs. Here we have used cryo-electron microscopy to solve the solution structure of native 15-PGDH and of 15-PGDH individually complexed with two distinct chemical inhibitors. These structures identify key 15-PGDH residues that mediate binding to both classes of inhibitors. Moreover, we identify a dynamic 15-PGDH lid domain that closes around the inhibitors, and that is likely fundamental to the physiologic 15-PGDH enzymatic mechanism. We furthermore identify two key residues, F185 and Y217, that act as hinges to regulate lid closing, and which both inhibitors exploit to capture the lid in the closed conformation, thus explaining their sub-nanomolar binding affinities. These findings provide the basis for further development of 15-PGDH targeted drugs as therapeutics for regenerative medicine. |
 External links External links |  Nat Commun / Nat Commun /  PubMed:36774348 / PubMed:36774348 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.4 - 3.3 Å |
| Structure data | EMDB-27010: CRYO-EM STRUCTURE OF HUMAN 15-PGDH IN COMPLEX WITH SMALL MOLECULE SW209415 EMDB-27025: CRYO-EM STRUCTURE OF HUMAN 15-PGDH IN COMPLEX WITH SMALL MOLECULE SW222746 EMDB-29005, PDB-8fd8: |
| Chemicals |  ChemComp-NAI: 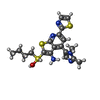 ChemComp-SWL: 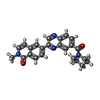 ChemComp-RLD: |
| Source |
|
 Keywords Keywords | OXIDOREDUCTASE / DEHYDROGENASE / INHIBITOR / OXIDOREDUCTASE-INHIBITOR complex |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers









 homo sapiens (human)
homo sapiens (human)