+Search query
-Structure paper
| Title | Electron cryotomography of Tula hantavirus suggests a unique assembly paradigm for enveloped viruses. |
|---|---|
| Journal, issue, pages | J Virol, Vol. 84, Issue 10, Page 4889-4897, Year 2010 |
| Publish date | Mar 10, 2010 |
 Authors Authors | Juha T Huiskonen / Jussi Hepojoki / Pasi Laurinmäki / Antti Vaheri / Hilkka Lankinen / Sarah J Butcher / Kay Grünewald /  |
| PubMed Abstract | Hantaviruses (family Bunyaviridae) are rodent-borne emerging viruses that cause a serious, worldwide threat to human health. Hantavirus diseases include hemorrhagic fever with renal syndrome and ...Hantaviruses (family Bunyaviridae) are rodent-borne emerging viruses that cause a serious, worldwide threat to human health. Hantavirus diseases include hemorrhagic fever with renal syndrome and hantavirus cardiopulmonary syndrome. Virions are enveloped and contain a tripartite single-stranded negative-sense RNA genome. Two types of glycoproteins, G(N) and G(C), are embedded in the viral membrane and form protrusions, or "spikes." The membrane encloses a ribonucleoprotein core, which consists of the RNA segments, the nucleocapsid protein, and the RNA-dependent RNA polymerase. Detailed information on hantavirus virion structure and glycoprotein spike composition is scarce. Here, we have studied the structures of Tula hantavirus virions using electron cryomicroscopy and tomography. Three-dimensional density maps show how the hantavirus surface glycoproteins, membrane, and ribonucleoprotein are organized. The structure of the G(N)-G(C) spike complex was solved to 3.6-nm resolution by averaging tomographic subvolumes. Each spike complex is a square-shaped assembly with 4-fold symmetry. Spike complexes formed ordered patches on the viral membrane by means of specific lateral interactions. These interactions may be sufficient for creating membrane curvature during virus budding. In conclusion, the structure and assembly principles of Tula hantavirus exemplify a unique assembly paradigm for enveloped viruses. |
 External links External links |  J Virol / J Virol /  PubMed:20219926 / PubMed:20219926 /  PubMed Central PubMed Central |
| Methods | EM (subtomogram averaging) |
| Resolution | 36.0 Å |
| Structure data | 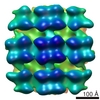 EMDB-1704: |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers



