+Search query
-Structure paper
| Title | Tetracenomycin X sequesters peptidyl-tRNA during translation of QK motifs. |
|---|---|
| Journal, issue, pages | Nat Chem Biol, Vol. 19, Issue 9, Page 1091-1096, Year 2023 |
| Publish date | Jun 15, 2023 |
 Authors Authors | Elodie C Leroy / Thomas N Perry / Thibaud T Renault / C Axel Innis /   |
| PubMed Abstract | As antimicrobial resistance threatens our ability to treat common bacterial infections, new antibiotics with limited cross-resistance are urgently needed. In this regard, natural products that target ...As antimicrobial resistance threatens our ability to treat common bacterial infections, new antibiotics with limited cross-resistance are urgently needed. In this regard, natural products that target the bacterial ribosome have the potential to be developed into potent drugs through structure-guided design, provided their mechanisms of action are well understood. Here we use inverse toeprinting coupled to next-generation sequencing to show that the aromatic polyketide tetracenomycin X primarily inhibits peptide bond formation between an incoming aminoacyl-tRNA and a terminal Gln-Lys (QK) motif in the nascent polypeptide. Using cryogenic electron microscopy, we reveal that translation inhibition at QK motifs occurs via an unusual mechanism involving sequestration of the 3' adenosine of peptidyl-tRNA in the drug-occupied nascent polypeptide exit tunnel of the ribosome. Our study provides mechanistic insights into the mode of action of tetracenomycin X on the bacterial ribosome and suggests a path forward for the development of novel aromatic polyketide antibiotics. |
 External links External links |  Nat Chem Biol / Nat Chem Biol /  PubMed:37322159 PubMed:37322159 |
| Methods | EM (single particle) |
| Resolution | 2.7 Å |
| Structure data | EMDB-14956, PDB-7zta: |
| Chemicals |  ChemComp-MG:  ChemComp-K:  ChemComp-ZN: 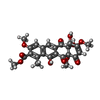 ChemComp-OCW: 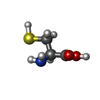 ChemComp-CYS: 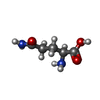 ChemComp-GLN:  ChemComp-HOH: |
| Source |
|
 Keywords Keywords | RIBOSOME / Antibiotic / Translation / Tetracenomycin X / Protein synthesis inhibitor |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers






