+Search query
-Structure paper
| Title | Structural basis of γ-secretase inhibition and modulation by small molecule drugs. |
|---|---|
| Journal, issue, pages | Cell, Vol. 184, Issue 2, Page 521-533.e14, Year 2021 |
| Publish date | Jan 21, 2021 |
 Authors Authors | Guanghui Yang / Rui Zhou / Xuefei Guo / Chuangye Yan / Jianlin Lei / Yigong Shi /  |
| PubMed Abstract | Development of γ-secretase inhibitors (GSIs) and modulators (GSMs) represents an attractive therapeutic opportunity for Alzheimer's disease (AD) and cancers. However, how these GSIs and GSMs target ...Development of γ-secretase inhibitors (GSIs) and modulators (GSMs) represents an attractive therapeutic opportunity for Alzheimer's disease (AD) and cancers. However, how these GSIs and GSMs target γ-secretase has remained largely unknown. Here, we report the cryoelectron microscopy (cryo-EM) structures of human γ-secretase bound individually to two GSI clinical candidates, Semagacestat and Avagacestat, a transition state analog GSI L685,458, and a classic GSM E2012, at overall resolutions of 2.6-3.1 Å. Remarkably, each of the GSIs occupies the same general location on presenilin 1 (PS1) that accommodates the β strand from amyloid precursor protein or Notch, interfering with substrate recruitment. L685,458 directly coordinates the two catalytic aspartate residues of PS1. E2012 binds to an allosteric site of γ-secretase on the extracellular side, potentially explaining its modulating activity. Structural analysis reveals a set of shared themes and variations for inhibitor and modulator recognition that will guide development of the next-generation substrate-selective inhibitors. |
 External links External links |  Cell / Cell /  PubMed:33373587 PubMed:33373587 |
| Methods | EM (single particle) |
| Resolution | 2.6 - 3.1 Å |
| Structure data | EMDB-0944, PDB-6lqg: EMDB-0957, PDB-6lr4: EMDB-30312, PDB-7c9i: EMDB-30614, PDB-7d8x: |
| Chemicals |  ChemComp-NAG:  ChemComp-EN9:  ChemComp-PC1:  ChemComp-CLR: 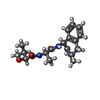 ChemComp-ESF: 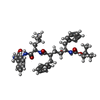 ChemComp-FTO: 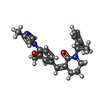 ChemComp-GZR:  ChemComp-PTY: |
| Source |
|
 Keywords Keywords | MEMBRANE PROTEIN/HYDROLASE / small molecule / MEMBRANE PROTEIN / MEMBRANE PROTEIN-HYDROLASE complex / Inhibitor / HYDROLASE / complex / modulator |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers











 homo sapiens (human)
homo sapiens (human)