+検索条件
-Structure paper
| タイトル | Ligand recognition, unconventional activation, and G protein coupling of the prostaglandin E receptor EP2 subtype. |
|---|---|
| ジャーナル・号・ページ | Sci Adv, Vol. 7, Issue 14, Year 2021 |
| 掲載日 | 2021年4月2日 |
 著者 著者 | Changxiu Qu / Chunyou Mao / Peng Xiao / Qingya Shen / Ya-Ni Zhong / Fan Yang / Dan-Dan Shen / Xiaona Tao / Huibing Zhang / Xu Yan / Ru-Jia Zhao / Junyan He / Ying Guan / Chao Zhang / Guihua Hou / Peng-Ju Zhang / Guige Hou / Zijian Li / Xiao Yu / Ren-Jie Chai / You-Fei Guan / Jin-Peng Sun / Yan Zhang /  |
| PubMed 要旨 | Selective modulation of the heterotrimeric G protein α S subunit-coupled prostaglandin E (PGE) receptor EP2 subtype is a promising therapeutic strategy for osteoporosis, ocular hypertension, ...Selective modulation of the heterotrimeric G protein α S subunit-coupled prostaglandin E (PGE) receptor EP2 subtype is a promising therapeutic strategy for osteoporosis, ocular hypertension, neurodegenerative diseases, and cardiovascular disorders. Here, we report the cryo-electron microscopy structure of the EP2-G complex with its endogenous agonist PGE and two synthesized agonists, taprenepag and evatanepag (CP-533536). These structures revealed distinct features of EP2 within the EP receptor family in terms of its unconventional receptor activation and G protein coupling mechanisms, including activation in the absence of a typical W "toggle switch" and coupling to G via helix 8. Moreover, inspection of the agonist-bound EP2 structures uncovered key motifs governing ligand selectivity. Our study provides important knowledge for agonist recognition and activation mechanisms of EP2 and will facilitate the rational design of drugs targeting the PGE signaling system. |
 リンク リンク |  Sci Adv / Sci Adv /  PubMed:33811074 / PubMed:33811074 /  PubMed Central PubMed Central |
| 手法 | EM (単粒子) |
| 解像度 | 2.8 - 2.9 Å |
| 構造データ | EMDB-30489, PDB-7cx2: EMDB-30490, PDB-7cx3: EMDB-30491, PDB-7cx4: |
| 化合物 | 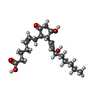 ChemComp-P2E:  ChemComp-HOH: 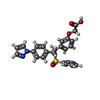 ChemComp-GNO: 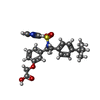 ChemComp-GM9: |
| 由来 |
|
 キーワード キーワード | MEMBRANE PROTEIN / GPCR / EP2 / Complex / PGE2 / Taprenepag / Evatanepag |
 ムービー
ムービー コントローラー
コントローラー 構造ビューア
構造ビューア 万見文献について
万見文献について









 homo sapiens (ヒト)
homo sapiens (ヒト)