+検索条件
-Structure paper
| タイトル | Large-scale movement of eIF3 domains during translation initiation modulate start codon selection. |
|---|---|
| ジャーナル・号・ページ | Nucleic Acids Res, Vol. 49, Issue 20, Page 11491-11511, Year 2021 |
| 掲載日 | 2021年11月18日 |
 著者 著者 | Jose L Llácer / Tanweer Hussain / Jinsheng Dong / Laura Villamayor / Yuliya Gordiyenko / Alan G Hinnebusch /     |
| PubMed 要旨 | The eukaryotic initiation factor 3 (eIF3) complex is involved in every step of translation initiation, but there is limited understanding of its molecular functions. Here, we present a single ...The eukaryotic initiation factor 3 (eIF3) complex is involved in every step of translation initiation, but there is limited understanding of its molecular functions. Here, we present a single particle electron cryomicroscopy (cryo-EM) reconstruction of yeast 48S ribosomal preinitiation complex (PIC) in an open conformation conducive to scanning, with core subunit eIF3b bound on the 40S interface near the decoding center in contact with the ternary complex eIF2·GTP·initiator tRNA. eIF3b is relocated together with eIF3i from their solvent interface locations observed in other PIC structures, with eIF3i lacking 40S contacts. Re-processing of micrographs of our previous 48S PIC in a closed state also suggests relocation of the entire eIF3b-3i-3g-3a-Cter module during the course of initiation. Genetic analysis indicates that high fidelity initiation depends on eIF3b interactions at the 40S subunit interface that promote the closed PIC conformation, or facilitate the relocation of eIF3b/eIF3i to the solvent interface, on start codon selection. |
 リンク リンク |  Nucleic Acids Res / Nucleic Acids Res /  PubMed:34648019 / PubMed:34648019 /  PubMed Central PubMed Central |
| 手法 | EM (単粒子) |
| 解像度 | 5.15 - 5.75 Å |
| 構造データ | |
| 化合物 | 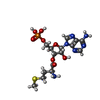 ChemComp-7NO:  ChemComp-MG:  ChemComp-ZN: 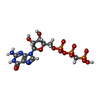 ChemComp-GCP: 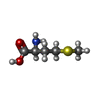 ChemComp-MET: |
| 由来 |
|
 キーワード キーワード | RIBOSOME / translation / initiation factors / 40S / eIF1A / eIF3 / eIF2 / tRNAi / 48S PIC / small ribosome subunit |
 ムービー
ムービー コントローラー
コントローラー 構造ビューア
構造ビューア 万見文献について
万見文献について







 kluyveromyces lactis (strain atcc 8585 / cbs 2359 / dsm 70799 / nbrc 1267 / nrrl y-1140 / wm37) (酵母)
kluyveromyces lactis (strain atcc 8585 / cbs 2359 / dsm 70799 / nbrc 1267 / nrrl y-1140 / wm37) (酵母)