+Search query
-Structure paper
| Title | Structural bases for aspartate recognition and polymerization efficiency of cyanobacterial cyanophycin synthetase. |
|---|---|
| Journal, issue, pages | Nat Commun, Vol. 13, Issue 1, Page 5097, Year 2022 |
| Publish date | Aug 30, 2022 |
 Authors Authors | Takuya Miyakawa / Jian Yang / Masato Kawasaki / Naruhiko Adachi / Ayumu Fujii / Yumiko Miyauchi / Tomonari Muramatsu / Toshio Moriya / Toshiya Senda / Masaru Tanokura /   |
| PubMed Abstract | Cyanophycin is a natural biopolymer consisting of equimolar amounts of aspartate and arginine as the backbone and branched sidechain, respectively. It is produced by a single enzyme, cyanophycin ...Cyanophycin is a natural biopolymer consisting of equimolar amounts of aspartate and arginine as the backbone and branched sidechain, respectively. It is produced by a single enzyme, cyanophycin synthetase (CphA1), and accumulates as a nitrogen reservoir during N fixation by most cyanobacteria. A recent structural study showed that three constituent domains of CphA1 function as two distinct catalytic sites and an oligomerization interface in cyanophycin synthesis. However, it remains unclear how the ATP-dependent addition of aspartate to cyanophycin is initiated at the catalytic site of the glutathione synthetase-like domain. Here, we report the cryogenic electron microscopy structures of CphA1, including a complex with aspartate, cyanophycin primer peptide, and ATP analog. These structures reveal the aspartate binding mode and phosphate-binding loop movement to the active site required for the reaction. Furthermore, structural and mutational data show a potential role of protein dynamics in the catalytic efficiency of the arginine condensation reaction. |
 External links External links |  Nat Commun / Nat Commun /  PubMed:36042318 / PubMed:36042318 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.52 - 2.96 Å |
| Structure data | EMDB-32381, PDB-7wac: EMDB-32382, PDB-7wad: EMDB-32383, PDB-7wae: EMDB-32384, PDB-7waf: |
| Chemicals |  ChemComp-MG:  ChemComp-AGS: 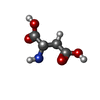 ChemComp-ASP: 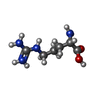 ChemComp-ARG: |
| Source |
|
 Keywords Keywords | LIGASE / Cyanophycin / Non-ribosomal peptide synthesis / ATP / Aspartate / Arginine |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers











 trichodesmium erythraeum ims101 (bacteria)
trichodesmium erythraeum ims101 (bacteria)