+Search query
-Structure paper
| Title | The mechanism of the amidases: mutating the glutamate adjacent to the catalytic triad inactivates the enzyme due to substrate mispositioning. |
|---|---|
| Journal, issue, pages | J. Biol. Chem., Vol. 288, Page 28514-28523, Year 2013 |
| Publish date | Sep 5, 2012 (structure data deposition date) |
 Authors Authors | Weber, B.W. / Kimani, S.W. / Varsani, A. / Cowan, D.A. / Hunter, R. / Venter, G.A. / Gumbart, J.C. / Sewell, B.T. |
 External links External links |  J. Biol. Chem. / J. Biol. Chem. /  PubMed:23946488 PubMed:23946488 |
| Methods | X-ray diffraction |
| Resolution | 1.1 - 1.9 Å |
| Structure data |  PDB-4gyl:  PDB-4gyn:  PDB-4kzf:  PDB-4lf0: |
| Chemicals | 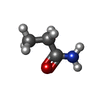 ChemComp-ROP:  ChemComp-CL:  ChemComp-HOH: |
| Source |
|
 Keywords Keywords | HYDROLASE / amidase / catalytic tetrad / amidase mechanism / acrylamide / Michael Adduct / active site / chloride ion / cysteine 166 oxidation / alpha-beta-beta-alpha sandwich |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers




