+Search query
-Structure paper
| Title | Increased hydrophobic interactions of iclaprim with Staphylococcus aureus dihydrofolate reductase are responsible for the increase in affinity and antibacterial activity |
|---|---|
| Journal, issue, pages | J. Antimicrob. Chemother., Vol. 63, Page 687-698, Year 2009 |
| Publish date | Jan 8, 2009 (structure data deposition date) |
 Authors Authors | Oefner, C. / Bandera, M. / Haldimann, A. / Laue, H. / Schulz, H. / Mukhija, S. / Parisi, S. / Weiss, L. / Lociuro, S. / Dale, G.E. |
 External links External links |  J. Antimicrob. Chemother. / J. Antimicrob. Chemother. /  PubMed:19211577 PubMed:19211577 |
| Methods | X-ray diffraction |
| Resolution | 2 - 2.35 Å |
| Structure data |  PDB-3fra:  PDB-3frb:  PDB-3frd:  PDB-3fre:  PDB-3frf: |
| Chemicals |  ChemComp-NAP: 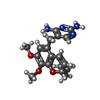 ChemComp-I2H:  ChemComp-HOH: 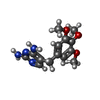 ChemComp-TOP:  ChemComp-NDP: 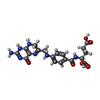 ChemComp-DHF: 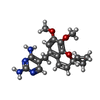 ChemComp-XCF: |
| Source |
|
 Keywords Keywords | OXIDOREDUCTASE / DHFR / NADP / One-carbon metabolism / S. aureus DHFR / S aureus DHFR |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers




