+Search query
-Structure paper
| Title | Structures, functions and adaptations of the human LINE-1 ORF2 protein. |
|---|---|
| Journal, issue, pages | Nature, Vol. 626, Issue 7997, Page 194-206, Year 2024 |
| Publish date | Dec 14, 2023 |
 Authors Authors | Eric T Baldwin / Trevor van Eeuwen / David Hoyos / Arthur Zalevsky / Egor P Tchesnokov / Roberto Sánchez / Bryant D Miller / Luciano H Di Stefano / Francesc Xavier Ruiz / Matthew Hancock / Esin Işik / Carlos Mendez-Dorantes / Thomas Walpole / Charles Nichols / Paul Wan / Kirsi Riento / Rowan Halls-Kass / Martin Augustin / Alfred Lammens / Anja Jestel / Paula Upla / Kera Xibinaku / Samantha Congreve / Maximiliaan Hennink / Kacper B Rogala / Anna M Schneider / Jennifer E Fairman / Shawn M Christensen / Brian Desrosiers / Gregory S Bisacchi / Oliver L Saunders / Nafeeza Hafeez / Wenyan Miao / Rosana Kapeller / Dennis M Zaller / Andrej Sali / Oliver Weichenrieder / Kathleen H Burns / Matthias Götte / Michael P Rout / Eddy Arnold / Benjamin D Greenbaum / Donna L Romero / John LaCava / Martin S Taylor /      |
| PubMed Abstract | The LINE-1 (L1) retrotransposon is an ancient genetic parasite that has written around one-third of the human genome through a 'copy and paste' mechanism catalysed by its multifunctional enzyme, open ...The LINE-1 (L1) retrotransposon is an ancient genetic parasite that has written around one-third of the human genome through a 'copy and paste' mechanism catalysed by its multifunctional enzyme, open reading frame 2 protein (ORF2p). ORF2p reverse transcriptase (RT) and endonuclease activities have been implicated in the pathophysiology of cancer, autoimmunity and ageing, making ORF2p a potential therapeutic target. However, a lack of structural and mechanistic knowledge has hampered efforts to rationally exploit it. We report structures of the human ORF2p 'core' (residues 238-1061, including the RT domain) by X-ray crystallography and cryo-electron microscopy in several conformational states. Our analyses identified two previously undescribed folded domains, extensive contacts to RNA templates and associated adaptations that contribute to unique aspects of the L1 replication cycle. Computed integrative structural models of full-length ORF2p show a dynamic closed-ring conformation that appears to open during retrotransposition. We characterize ORF2p RT inhibition and reveal its underlying structural basis. Imaging and biochemistry show that non-canonical cytosolic ORF2p RT activity can produce RNA:DNA hybrids, activating innate immune signalling through cGAS/STING and resulting in interferon production. In contrast to retroviral RTs, L1 RT is efficiently primed by short RNAs and hairpins, which probably explains cytosolic priming. Other biochemical activities including processivity, DNA-directed polymerization, non-templated base addition and template switching together allow us to propose a revised L1 insertion model. Finally, our evolutionary analysis demonstrates structural conservation between ORF2p and other RNA- and DNA-dependent polymerases. We therefore provide key mechanistic insights into L1 polymerization and insertion, shed light on the evolutionary history of L1 and enable rational drug development targeting L1. |
 External links External links |  Nature / Nature /  PubMed:38096902 / PubMed:38096902 /  PubMed Central PubMed Central |
| Methods | EM (single particle) / X-ray diffraction |
| Resolution | 2.1 - 4.06 Å |
| Structure data |  EMDB-40856: Single particle reconstruction of the human LINE-1 ORF2p without substrate (apo) EMDB-40858, PDB-8sxt: EMDB-40859, PDB-8sxu:  PDB-8c8j: |
| Chemicals |  ChemComp-CIT:  ChemComp-TTP:  ChemComp-EDO:  ChemComp-DMS: 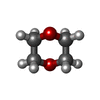 ChemComp-DIO:  ChemComp-MG:  ChemComp-CL:  ChemComp-HOH: |
| Source |
|
 Keywords Keywords | REPLICATION / REVERSE TRANSCRIPTASE / RT / DTTP / HYRBID DUPLEX / TEMPLATE PRIMER / RNA BINDING PROTEIN/RNA/DNA / LINE-1 / RNA BINDING PROTEIN / RNA BINDING PROTEIN-RNA-DNA complex / RNA BINDING PROTEIN/RNA / RNA BINDING PROTEIN-RNA complex |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers







 homo sapiens (human)
homo sapiens (human)