+Search query
-Structure paper
| Title | Cryo-EM structures of human GPR34 enable the identification of selective antagonists. |
|---|---|
| Journal, issue, pages | Proc Natl Acad Sci U S A, Vol. 120, Issue 39, Page e2308435120, Year 2023 |
| Publish date | Sep 26, 2023 |
 Authors Authors | Anjie Xia / Xihao Yong / Changbin Zhang / Guifeng Lin / Guowen Jia / Chang Zhao / Xin Wang / Yize Hao / Yifei Wang / Pei Zhou / Xin Yang / Yue Deng / Chao Wu / Yujiao Chen / Jiawei Zhu / Xiaodi Tang / Jingming Liu / Shiyu Zhang / Jiahao Zhang / Zheng Xu / Qian Hu / Jinlong Zhao / Yuting Yue / Wei Yan / Zhaoming Su / Yuquan Wei / Rongbin Zhou / Haohao Dong / Zhenhua Shao / Shengyong Yang /  |
| PubMed Abstract | GPR34 is a functional G-protein-coupled receptor of Lysophosphatidylserine (LysoPS), and has pathogenic roles in numerous diseases, yet remains poorly targeted. We herein report a cryo-electron ...GPR34 is a functional G-protein-coupled receptor of Lysophosphatidylserine (LysoPS), and has pathogenic roles in numerous diseases, yet remains poorly targeted. We herein report a cryo-electron microscopy (cryo-EM) structure of GPR34 bound with LysoPS (18:1) and G protein, revealing a unique ligand recognition mode with the negatively charged head group of LysoPS occupying a polar cavity formed by TM3, 6 and 7, and the hydrophobic tail of LysoPS residing in a lateral open hydrophobic groove formed by TM3-5. Virtual screening and subsequent structural optimization led to the identification of a highly potent and selective antagonist (YL-365). Design of fusion proteins allowed successful determination of the challenging cryo-EM structure of the inactive GPR34 complexed with YL-365, which revealed the competitive binding of YL-365 in a portion of the orthosteric binding pocket of GPR34 and the antagonist-binding-induced allostery in the receptor, implicating the inhibition mechanism of YL-365. Moreover, YL-365 displayed excellent activity in a neuropathic pain model without obvious toxicity. Collectively, this study offers mechanistic insights into the endogenous agonist recognition and antagonist inhibition of GPR34, and provides proof of concept that targeting GPR34 represents a promising strategy for disease treatment. |
 External links External links |  Proc Natl Acad Sci U S A / Proc Natl Acad Sci U S A /  PubMed:37733739 / PubMed:37733739 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 3.12 - 3.34 Å |
| Structure data | EMDB-35832, PDB-8iyx:  EMDB-35870: Cryo-EM map of human GPR34  EMDB-35871: Cryo-EM map of Gi-scFv16 complex EMDB-40270, PDB-8sai: |
| Chemicals | 
ChemComp-S6R: 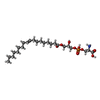 ChemComp-S12: |
| Source |
|
 Keywords Keywords | MEMBRANE PROTEIN/INHIBITOR / Inhibitor / GPR34 receptor in complex with the antagonist YL-365 / MEMBRANE PROTEIN / MEMBRANE PROTEIN-INHIBITOR complex / LIPID BINDING PROTEIN / Complex / Agonist |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers







 homo sapiens (human)
homo sapiens (human)
