+Search query
-Structure paper
| Title | Conformational rearrangements of SV40 large T antigen during early replication events. |
|---|---|
| Journal, issue, pages | J Mol Biol, Vol. 397, Issue 5, Page 1276-1286, Year 2010 |
| Publish date | Apr 16, 2010 |
 Authors Authors | Isabel Cuesta / Rafael Núñez-Ramírez / Sjors H W Scheres / Dahai Gai / Xiaojiang S Chen / Ellen Fanning / Jose María Carazo /  |
| PubMed Abstract | The Simian virus 40 (SV40) large tumor antigen (LTag) functions as the replicative helicase and initiator for viral DNA replication. For SV40 replication, the first essential step is the assembly of ...The Simian virus 40 (SV40) large tumor antigen (LTag) functions as the replicative helicase and initiator for viral DNA replication. For SV40 replication, the first essential step is the assembly of an LTag double hexamer at the origin DNA that will subsequently melt the origin DNA to initiate fork unwinding. In this study, we used three-dimensional cryo-electron microscopy to visualize early events in the activation of DNA replication in the SV40 model system. We obtained structures of wild-type double-hexamer complexes of LTag bound to SV40 origin DNA, to which atomic structures have been fitted. Wild-type LTag was observed in two distinct conformations: In one conformation, the central module containing the J-domains and the origin binding domains of both hexamers is a compact closed ring. In the other conformation, the central module is an open ring with a gap formed by rearrangement of the N-terminal regions of the two hexamers, potentially allowing for the passage of single-stranded DNA generated from the melted origin DNA. Double-hexamer complexes containing mutant LTag that lacks the N-terminal J-domain show the central module predominantly in the closed-ring state. Analyses of the LTag C-terminal regions reveal that the LTag hexamers bound to the A/T-rich tract origin of replication and early palindrome origin of replication elements are structurally distinct. Lastly, visualization of DNA density protruding from the LTag C-terminal domains suggests that oligomerization of the LTag complex takes place on double-stranded DNA. |
 External links External links |  J Mol Biol / J Mol Biol /  PubMed:20219473 / PubMed:20219473 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 19.0 - 30.0 Å |
| Structure data | 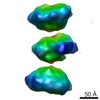 EMDB-1681:  EMDB-1682:  EMDB-1683: 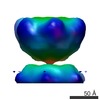 EMDB-1684:  EMDB-1685:  EMDB-1686:  EMDB-1687: |
| Source |
|
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers



 Simian virus 40
Simian virus 40