+Search query
-Structure paper
| Title | Structural insights into ligand recognition and selectivity of somatostatin receptors. |
|---|---|
| Journal, issue, pages | Cell Res, Vol. 32, Issue 8, Page 761-772, Year 2022 |
| Publish date | Jun 23, 2022 |
 Authors Authors | Wenli Zhao / Shuo Han / Na Qiu / Wenbo Feng / Mengjie Lu / Wenru Zhang / Mu Wang / Qingtong Zhou / Shutian Chen / Wei Xu / Juan Du / Xiaojing Chu / Cuiying Yi / Antao Dai / Liaoyuan Hu / Michelle Y Shen / Yaping Sun / Qing Zhang / Yingli Ma / Wenge Zhong / Dehua Yang / Ming-Wei Wang / Beili Wu / Qiang Zhao /  |
| PubMed Abstract | Somatostatin receptors (SSTRs) play versatile roles in inhibiting the secretion of multiple hormones such as growth hormone and thyroid-stimulating hormone, and thus are considered as targets for ...Somatostatin receptors (SSTRs) play versatile roles in inhibiting the secretion of multiple hormones such as growth hormone and thyroid-stimulating hormone, and thus are considered as targets for treating multiple tumors. Despite great progress made in therapeutic development against this diverse receptor family, drugs that target SSTRs still show limited efficacy with preferential binding affinity and conspicuous side-effects. Here, we report five structures of SSTR2 and SSTR4 in different states, including two crystal structures of SSTR2 in complex with a selective peptide antagonist and a non-peptide agonist, respectively, a cryo-electron microscopy (cryo-EM) structure of G-bound SSTR2 in the presence of the endogenous ligand SST-14, as well as two cryo-EM structures of G-bound SSTR4 in complex with SST-14 and a small-molecule agonist J-2156, respectively. By comparison of the SSTR structures in different states, molecular mechanisms of agonism and antagonism were illustrated. Together with computational and functional analyses, the key determinants responsible for ligand recognition and selectivity of different SSTR subtypes and multiform binding modes of peptide and non-peptide ligands were identified. Insights gained in this study will help uncover ligand selectivity of various SSTRs and accelerate the development of new molecules with better efficacy by targeting SSTRs. |
 External links External links |  Cell Res / Cell Res /  PubMed:35739238 / PubMed:35739238 /  PubMed Central PubMed Central |
| Methods | EM (single particle) / X-ray diffraction |
| Resolution | 2.6 - 3.1 Å |
| Structure data | EMDB-33302, PDB-7xmr: EMDB-33303, PDB-7xms: EMDB-33304, PDB-7xmt:  PDB-7xn9:  PDB-7xna: |
| Chemicals | 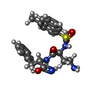 ChemComp-I8B:  ChemComp-EPE: 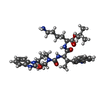 ChemComp-GI9: |
| Source |
|
 Keywords Keywords | SIGNALING PROTEIN / G protein-coupled receptor / somatostatin receptor 2 / cryo-EM / somatostatin receptor 4 / STRUCTURAL PROTEIN / SIGNARING PROTEIN/INHIBITOR / SIGNARING PROTEIN-INHIBITOR complex / SIGNALING PROTEIN/INHIBITOR / SIGNALING PROTEIN-INHIBITOR complex |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers









 homo sapiens (human)
homo sapiens (human)
 niallia circulans (bacteria)
niallia circulans (bacteria)