+検索条件
-Structure paper
| タイトル | The structural basis for the phospholipid remodeling by lysophosphatidylcholine acyltransferase 3. |
|---|---|
| ジャーナル・号・ページ | Nat Commun, Vol. 12, Issue 1, Page 6869, Year 2021 |
| 掲載日 | 2021年11月25日 |
 著者 著者 | Qing Zhang / Deqiang Yao / Bing Rao / Liyan Jian / Yang Chen / Kexin Hu / Ying Xia / Shaobai Li / Yafeng Shen / An Qin / Jie Zhao / Lu Zhou / Ming Lei / Xian-Cheng Jiang / Yu Cao /   |
| PubMed 要旨 | As the major component of cell membranes, phosphatidylcholine (PC) is synthesized de novo in the Kennedy pathway and then undergoes extensive deacylation-reacylation remodeling via Lands' cycle. The ...As the major component of cell membranes, phosphatidylcholine (PC) is synthesized de novo in the Kennedy pathway and then undergoes extensive deacylation-reacylation remodeling via Lands' cycle. The re-acylation is catalyzed by lysophosphatidylcholine acyltransferase (LPCAT) and among the four LPCAT members in human, the LPCAT3 preferentially introduces polyunsaturated acyl onto the sn-2 position of lysophosphatidylcholine, thereby modulating the membrane fluidity and membrane protein functions therein. Combining the x-ray crystallography and the cryo-electron microscopy, we determined the structures of LPCAT3 in apo-, acyl donor-bound, and acyl receptor-bound states. A reaction chamber was revealed in the LPCAT3 structure where the lysophosphatidylcholine and arachidonoyl-CoA were positioned in two tunnels connected near to the catalytic center. A side pocket was found expanding the tunnel for the arachidonoyl CoA and holding the main body of arachidonoyl. The structural and functional analysis provides the basis for the re-acylation of lysophosphatidylcholine and the substrate preference during the reactions. |
 リンク リンク |  Nat Commun / Nat Commun /  PubMed:34824256 / PubMed:34824256 /  PubMed Central PubMed Central |
| 手法 | EM (単粒子) / X線回折 |
| 解像度 | 3.4 - 3.57 Å |
| 構造データ | EMDB-31442, PDB-7f3x: EMDB-31443, PDB-7f40:  PDB-7ewt: |
| 化合物 | 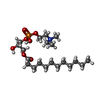 ChemComp-LAP: 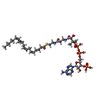 ChemComp-3IX:  ChemComp-PCW: |
| 由来 |
|
 キーワード キーワード | TRANSFERASE / Lysophospholipid Acyltransferase / membrane bound O-acyltransferase / phospholipid remodeling / MEMBRANE PROTEIN / LPCAT3 / membrane-bound O-acyltransferase / cryo-EM |
 ムービー
ムービー コントローラー
コントローラー 構造ビューア
構造ビューア 万見文献について
万見文献について








