+Search query
-Structure paper
| Title | Structures of prokaryotic ubiquitin-like protein Pup in complex with depupylase Dop reveal the mechanism of catalytic phosphate formation. |
|---|---|
| Journal, issue, pages | Nat Commun, Vol. 12, Page 6635-6635, Year 2021 |
| Publish date | Jun 23, 2021 (structure data deposition date) |
 Authors Authors | Cui, H. / Muller, A.U. / Leibundgut, M. / Tian, J. / Ban, N. / Weber-Ban, E. |
 External links External links |  Nat Commun / Nat Commun /  PubMed:34789727 PubMed:34789727 |
| Methods | X-ray diffraction |
| Resolution | 1.394 - 1.88 Å |
| Structure data |  PDB-7oxv:  PDB-7oxy:  PDB-7oy3:  PDB-7oyf:  PDB-7oyh: |
| Chemicals |  ChemComp-PGE:  ChemComp-SCN:  ChemComp-PEG:  ChemComp-EDO:  ChemComp-HOH:  ChemComp-MG:  ChemComp-ACP:  ChemComp-ACT:  ChemComp-K:  ChemComp-ADP: 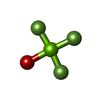 ChemComp-KQB:  ChemComp-SO4: 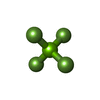 ChemComp-MF4: |
| Source |
|
 Keywords Keywords | HYDROLASE / depupylase / Dop-loop / deamidase / Pup / ATP hydrolysis / Pup binding / phosphorylated Pup binding / transition state / product state |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers



 acidothermus cellulolyticus (bacteria)
acidothermus cellulolyticus (bacteria)