+Search query
-Structure paper
| Title | A structure of human Scap bound to Insig-2 suggests how their interaction is regulated by sterols. |
|---|---|
| Journal, issue, pages | Science, Vol. 371, Issue 6533, Year 2021 |
| Publish date | Mar 5, 2021 |
 Authors Authors | Renhong Yan / Pingping Cao / Wenqi Song / Hongwu Qian / Ximing Du / Hudson W Coates / Xin Zhao / Yaning Li / Shuai Gao / Xin Gong / Ximing Liu / Jianhua Sui / Jianlin Lei / Hongyuan Yang / Andrew J Brown / Qiang Zhou / Chuangye Yan / Nieng Yan /    |
| PubMed Abstract | The sterol regulatory element-binding protein (SREBP) pathway controls cellular homeostasis of sterols. The key players in this pathway, Scap and Insig-1 and -2, are membrane-embedded sterol sensors. ...The sterol regulatory element-binding protein (SREBP) pathway controls cellular homeostasis of sterols. The key players in this pathway, Scap and Insig-1 and -2, are membrane-embedded sterol sensors. The 25-hydroxycholesterol (25HC)-dependent association of Scap and Insig acts as the master switch for the SREBP pathway. Here, we present cryo-electron microscopy analysis of the human Scap and Insig-2 complex in the presence of 25HC, with the transmembrane (TM) domains determined at an average resolution of 3.7 angstrom. The sterol-sensing domain in Scap and all six TMs in Insig-2 were resolved. A 25HC molecule is sandwiched between the S4 to S6 segments in Scap and TMs 3 and 4 in Insig-2 in the luminal leaflet of the membrane. Unwinding of the middle of the Scap-S4 segment is crucial for 25HC binding and Insig association. |
 External links External links |  Science / Science /  PubMed:33446483 PubMed:33446483 |
| Methods | EM (single particle) |
| Resolution | 3.7 Å |
| Structure data | EMDB-30074, PDB-6m49: |
| Chemicals | 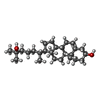 ChemComp-HC3: |
| Source |
|
 Keywords Keywords | MEMBRANE PROTEIN / Scap / Insig / cholesterol / sterol sensing / 25-hydroxycholesterol / SREBP |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers





 homo sapiens (human)
homo sapiens (human)