+検索条件
-Structure paper
| タイトル | High-resolution structures of multiple 5-HTR-setron complexes reveal a novel mechanism of competitive inhibition. |
|---|---|
| ジャーナル・号・ページ | Elife, Vol. 9, Year 2020 |
| 掲載日 | 2020年10月16日 |
 著者 著者 | Sandip Basak / Arvind Kumar / Steven Ramsey / Eric Gibbs / Abhijeet Kapoor / Marta Filizola / Sudha Chakrapani /  |
| PubMed 要旨 | Serotonin receptors (5-HTR) play a crucial role in regulating gut movement, and are the principal target of setrons, a class of high-affinity competitive antagonists, used in the management of nausea ...Serotonin receptors (5-HTR) play a crucial role in regulating gut movement, and are the principal target of setrons, a class of high-affinity competitive antagonists, used in the management of nausea and vomiting associated with radiation and chemotherapies. Structural insights into setron-binding poses and their inhibitory mechanisms are just beginning to emerge. Here, we present high-resolution cryo-EM structures of full-length 5-HTR in complex with palonosetron, ondansetron, and alosetron. Molecular dynamic simulations of these structures embedded in a fully-hydrated lipid environment assessed the stability of ligand-binding poses and drug-target interactions over time. Together with simulation results of apo- and serotonin-bound 5-HTR, the study reveals a distinct interaction fingerprint between the various setrons and binding-pocket residues that may underlie their diverse affinities. In addition, varying degrees of conformational change in the setron-5-HTR structures, throughout the channel and particularly along the channel activation pathway, suggests a novel mechanism of competitive inhibition. |
 リンク リンク |  Elife / Elife /  PubMed:33063666 / PubMed:33063666 /  PubMed Central PubMed Central |
| 手法 | EM (単粒子) |
| 解像度 | 2.92 - 3.35 Å |
| 構造データ | EMDB-21511, PDB-6w1j: EMDB-21512, PDB-6w1m: EMDB-21518, PDB-6w1y: |
| 化合物 | 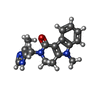 ChemComp-S7Y:  ChemComp-CL:  ChemComp-S87: 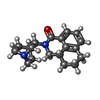 ChemComp-O7B: |
| 由来 |
|
 キーワード キーワード | TRANSPORT PROTEIN |
 ムービー
ムービー コントローラー
コントローラー 構造ビューア
構造ビューア 万見文献について
万見文献について










