+Search query
-Structure paper
| Title | Structural basis for pharmacological modulation of the TRPC6 channel. |
|---|---|
| Journal, issue, pages | Elife, Vol. 9, Year 2020 |
| Publish date | Mar 9, 2020 |
 Authors Authors | Yonghong Bai / Xinchao Yu / Hao Chen / Daniel Horne / Ryan White / Xiaosu Wu / Paul Lee / Yan Gu / Sudipa Ghimire-Rijal / Daniel C-H Lin / Xin Huang /  |
| PubMed Abstract | Transient receptor potential canonical (TRPC) proteins form nonselective cation channels that play physiological roles in a wide variety of cells. Despite growing evidence supporting the therapeutic ...Transient receptor potential canonical (TRPC) proteins form nonselective cation channels that play physiological roles in a wide variety of cells. Despite growing evidence supporting the therapeutic potential of TRPC6 inhibition in treating pathological cardiac and renal conditions, mechanistic understanding of TRPC6 function and modulation remains obscure. Here we report cryo-EM structures of TRPC6 in both antagonist-bound and agonist-bound states. The structures reveal two novel recognition sites for the small-molecule modulators corroborated by mutagenesis data. The antagonist binds to a cytoplasm-facing pocket formed by S1-S4 and the TRP helix, whereas the agonist wedges at the subunit interface between S6 and the pore helix. Conformational changes upon ligand binding illuminate a mechanistic rationale for understanding TRPC6 modulation. Furthermore, structural and mutagenesis analyses suggest several disease-related mutations enhance channel activity by disrupting interfacial interactions. Our results provide principles of drug action that may facilitate future design of small molecules to ameliorate TRPC6-mediated diseases. |
 External links External links |  Elife / Elife /  PubMed:32149605 / PubMed:32149605 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.84 - 3.08 Å |
| Structure data | EMDB-20953, PDB-6uz8: EMDB-20954, PDB-6uza: |
| Chemicals |  ChemComp-SBM:  ChemComp-Y01: 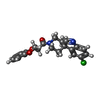 ChemComp-R0D: 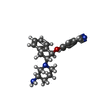 ChemComp-R0G: 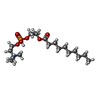 ChemComp-S9Y: 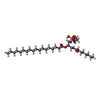 ChemComp-SBJ: |
| Source |
|
 Keywords Keywords | TRANSPORT PROTEIN / TRP channel / Agonist / Antagonist |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers







 homo sapiens (human)
homo sapiens (human)