+検索条件
-Structure paper
| タイトル | A native-like SOSIP.664 trimer based on an HIV-1 subtype B env gene. |
|---|---|
| ジャーナル・号・ページ | J Virol, Vol. 89, Issue 6, Page 3380-3395, Year 2015 |
| 掲載日 | 2015年1月14日 |
 著者 著者 | Pavel Pugach / Gabriel Ozorowski / Albert Cupo / Rajesh Ringe / Anila Yasmeen / Natalia de Val / Ronald Derking / Helen J Kim / Jacob Korzun / Michael Golabek / Kevin de Los Reyes / Thomas J Ketas / Jean-Philippe Julien / Dennis R Burton / Ian A Wilson / Rogier W Sanders / P J Klasse / Andrew B Ward / John P Moore /   |
| PubMed 要旨 | Recombinant trimeric mimics of the human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein (Env) spike should expose as many epitopes as possible for broadly neutralizing antibodies (bNAbs) ...Recombinant trimeric mimics of the human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein (Env) spike should expose as many epitopes as possible for broadly neutralizing antibodies (bNAbs) but few, if any, for nonneutralizing antibodies (non-NAbs). Soluble, cleaved SOSIP.664 gp140 trimers based on the subtype A strain BG505 approach this ideal and are therefore plausible vaccine candidates. Here, we report on the production and in vitro properties of a new SOSIP.664 trimer derived from a subtype B env gene, B41, including how to make this protein in low-serum media without proteolytic damage (clipping) to the V3 region. We also show that nonclipped trimers can be purified successfully via a positive-selection affinity column using the bNAb PGT145, which recognizes a quaternary structure-dependent epitope at the trimer apex. Negative-stain electron microscopy imaging shows that the purified, nonclipped, native-like B41 SOSIP.664 trimers contain two subpopulations, which we propose represent an equilibrium between the fully closed and a more open conformation. The latter is different from the fully open, CD4 receptor-bound conformation and may represent an intermediate state of the trimer. This new subtype B trimer adds to the repertoire of native-like Env proteins that are suitable for immunogenicity and structural studies. IMPORTANCE: The cleaved, trimeric envelope protein complex is the only neutralizing antibody target on the HIV-1 surface. Many vaccine strategies are based on inducing neutralizing antibodies. For HIV-1, one approach involves using recombinant, soluble protein mimics of the native trimer. At present, the only reliable way to make native-like, soluble trimers in practical amounts is via the introduction of specific sequence changes that confer stability on the cleaved form of Env. The resulting proteins are known as SOSIP.664 gp140 trimers, and the current paradigm is based on the BG505 subtype A env gene. Here, we describe the production and characterization of a SOSIP.664 protein derived from a subtype B gene (B41), together with a simple, one-step method to purify native-like trimers by affinity chromatography with a trimer-specific bNAb, PGT145. The resulting trimers will be useful for structural and immunogenicity experiments aimed at devising ways to make an effective HIV-1 vaccine. |
 リンク リンク |  J Virol / J Virol /  PubMed:25589637 / PubMed:25589637 /  PubMed Central PubMed Central |
| 手法 | EM (単粒子) |
| 解像度 | 17.0 Å |
| 構造データ | 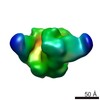 EMDB-6256: |
| 由来 |
|
 ムービー
ムービー コントローラー
コントローラー 構造ビューア
構造ビューア 万見文献について
万見文献について



 Simian-Human immunodeficiency virus (ウイルス)
Simian-Human immunodeficiency virus (ウイルス) Homo sapiens (ヒト)
Homo sapiens (ヒト)