+Search query
-Structure paper
| Title | Quaternary structure of human, Drosophila melanogaster and Caenorhabditis elegans MFE-2 in solution from synchrotron small-angle X-ray scattering. |
|---|---|
| Journal, issue, pages | FEBS Lett, Vol. 587, Issue 4, Page 305-310, Year 2013 |
| Publish date | Feb 14, 2013 |
 Authors Authors | Maija L Mehtälä / Tatu J K Haataja / Clément E Blanchet / J Kalervo Hiltunen / Dmitri I Svergun / Tuomo Glumoff /  |
| PubMed Abstract | Multifunctional enzyme type 2 (MFE-2) forms part of the fatty acid β-oxidation pathway in peroxisomes. MFE-2s from various species reveal proteins with structurally homologous functional domains ...Multifunctional enzyme type 2 (MFE-2) forms part of the fatty acid β-oxidation pathway in peroxisomes. MFE-2s from various species reveal proteins with structurally homologous functional domains assembled in different compilations. Crystal structures of all domain types are known. SAXS data from human, fruit fly and Caenorhabditiselegans MFE-2s and their constituent domains were collected, and both ab initio and rigid body models constructed. Location of the putative substrate binding helper domain SCP-2L (sterol carrier protein 2-like), which is not part of MFE-2 protein in every species and not seen as part of any previous MFE-2 structures, was determined. The obtained models of human and C. elegans MFE-2 lend a direct structural support to the idea of the biological role of SCP-2L. |
 External links External links |  FEBS Lett / FEBS Lett /  PubMed:23313254 PubMed:23313254 |
| Methods | SAS (X-ray synchrotron) |
| Structure data |  SASDAF4: DmMfe2 (Peroxisomal multifunctional enzyme type 2) 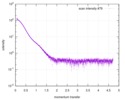 SASDAG4: HsMfe2 (Peroxisomal multifunctional enzyme type 2) |
| Source |
|
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers




 Homo sapiens (human)
Homo sapiens (human)