+検索条件
-Structure paper
| タイトル | Structural insights into the architecture and allostery of full-length AMP-activated protein kinase. |
|---|---|
| ジャーナル・号・ページ | Structure, Vol. 19, Issue 4, Page 515-522, Year 2011 |
| 掲載日 | 2011年4月13日 |
 著者 著者 | Li Zhu / Lei Chen / Xiao-Ming Zhou / Yuan-Yuan Zhang / Yi-Jiong Zhang / Jing Zhao / Shang-Rong Ji / Jia-Wei Wu / Yi Wu /  |
| PubMed 要旨 | AMP-activated protein kinase (AMPK) is a heterotrimeric complex composed of α catalytic subunit, β scaffolding subunit, and γ regulatory subunit with critical roles in maintaining cellular energy ...AMP-activated protein kinase (AMPK) is a heterotrimeric complex composed of α catalytic subunit, β scaffolding subunit, and γ regulatory subunit with critical roles in maintaining cellular energy homeostasis. However, the molecular architecture of the intact complex and the allostery associated with the adenosine binding-induced regulation of kinase activity remain unclear. Here, we determine the three-dimensional reconstruction and subunit organization of the full-length rat AMPK (α1β1γ1) through single-particle electron-microscopy. By comparing the structures of AMPK in ATP- and AMP-bound states, we are able to visualize the sequential conformational changes underlying kinase activation that transmits from the adenosine binding sites in the γ subunit to the kinase domain of the α subunit. These results not only make substantial revision to the current model of AMPK assembly, but also highlight a central role of the linker sequence of the α subunit in mediating the allostery of AMPK. |
 リンク リンク |  Structure / Structure /  PubMed:21481774 PubMed:21481774 |
| 手法 | EM (単粒子) |
| 解像度 | 19.0 - 20.0 Å |
| 構造データ | 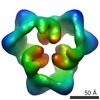 EMDB-1897: 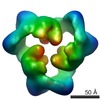 EMDB-1898: 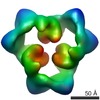 EMDB-1899: |
| 由来 |
|
 ムービー
ムービー コントローラー
コントローラー 構造ビューア
構造ビューア 万見文献について
万見文献について




