[English] 日本語
 Yorodumi
Yorodumi- EMDB-6871: A microtubule-dynein tethering complex regulates the axonemal inn... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-6871 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | A microtubule-dynein tethering complex regulates the axonemal inner dynein f (I1) | |||||||||
 Map data Map data | Chlamydomonas fap44 mutant, IDA f in ATP plus vanadate state (state III) | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  | |||||||||
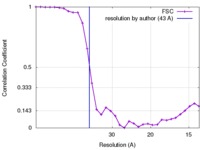
| Method | subtomogram averaging / cryo EM / Resolution: 43.0 Å | |||||||||
 Authors Authors | Kubo T / Hou Y / Cochran DA / Witman GB / Oda T | |||||||||
 Citation Citation |  Journal: Mol Biol Cell / Year: 2018 Journal: Mol Biol Cell / Year: 2018Title: A microtubule-dynein tethering complex regulates the axonemal inner dynein f (I1). Authors: Tomohiro Kubo / Yuqing Hou / Deborah A Cochran / George B Witman / Toshiyuki Oda /   Abstract: Motility of cilia/flagella is generated by a coordinated activity of thousands of dyneins. Inner dynein arms (IDAs) are particularly important for the formation of ciliary/flagellar waveforms, but ...Motility of cilia/flagella is generated by a coordinated activity of thousands of dyneins. Inner dynein arms (IDAs) are particularly important for the formation of ciliary/flagellar waveforms, but the molecular mechanism of IDA regulation is poorly understood. Here we show using cryoelectron tomography and biochemical analyses of Chlamydomonas flagella that a conserved protein FAP44 forms a complex that tethers IDA f (I1 dynein) head domains to the A-tubule of the axonemal outer doublet microtubule. In wild-type flagella, IDA f showed little nucleotide-dependent movement except for a tilt in the f β head perpendicular to the microtubule-sliding direction. In the absence of the tether complex, however, addition of ATP and vanadate caused a large conformational change in the IDA f head domains, suggesting that the movement of IDA f is mechanically restricted by the tether complex. Motility defects in flagella missing the tether demonstrates the importance of the IDA f-tether interaction in the regulation of ciliary/flagellar beating. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_6871.map.gz emd_6871.map.gz | 46.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-6871-v30.xml emd-6871-v30.xml emd-6871.xml emd-6871.xml | 10.3 KB 10.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_6871_fsc.xml emd_6871_fsc.xml | 3 KB | Display |  FSC data file FSC data file |
| Images |  emd_6871.png emd_6871.png | 76.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-6871 http://ftp.pdbj.org/pub/emdb/structures/EMD-6871 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6871 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6871 | HTTPS FTP |
-Validation report
| Summary document |  emd_6871_validation.pdf.gz emd_6871_validation.pdf.gz | 78.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_6871_full_validation.pdf.gz emd_6871_full_validation.pdf.gz | 77.3 KB | Display | |
| Data in XML |  emd_6871_validation.xml.gz emd_6871_validation.xml.gz | 495 B | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6871 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6871 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6871 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6871 | HTTPS FTP |
-Related structure data
| Related structure data |  6866C  6867C  6868C  6869C  6870C  6872C  6873C  6874C C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_6871.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_6871.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Chlamydomonas fap44 mutant, IDA f in ATP plus vanadate state (state III) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 7.025 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Inner dynein f
| Entire | Name: Inner dynein f |
|---|---|
| Components |
|
-Supramolecule #1: Inner dynein f
| Supramolecule | Name: Inner dynein f / type: organelle_or_cellular_component / ID: 1 / Parent: 0 Details: fap44 mutant Chlamydomonas axoneme in ATP plus vanadate state (state III) |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Concentration | 0.02 mg/mL |
|---|---|
| Buffer | pH: 7.2 |
| Grid | Model: Homemade / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: blot 5.5 sec before plunging. |
- Electron microscopy
Electron microscopy
| Microscope | JEOL 3100FFC |
|---|---|
| Specialist optics | Energy filter - Name: In-column Omega Filter |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average exposure time: 6.0 sec. / Average electron dose: 1.6 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: GATAN 914 HIGH TILT LIQUID NITROGEN CRYO TRANSFER TOMOGRAPHY HOLDER Cooling holder cryogen: HELIUM |
 Movie
Movie Controller
Controller



 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)