[English] 日本語
 Yorodumi
Yorodumi- EMDB-5108: Feline panleukopenia virus in complex with FAb from neutralizing ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-5108 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Feline panleukopenia virus in complex with FAb from neutralizing antibody MAb 8 | |||||||||
 Map data Map data | Feline panleukopenia virus in complex with FAb from neutralizing antibody MAb 8 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | parvovirus / antigenic epitope / antibody / Fab / neutralizing | |||||||||
| Biological species |  Feline panleukopenia virus Feline panleukopenia virus | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 11.1 Å | |||||||||
 Authors Authors | Hafenstein S / Bowman VD / Sun T / Nelson CDS / Palermo LM / Chipman PR / Battisti AJ / Parrish CR / Rossmann MG | |||||||||
 Citation Citation |  Journal: J Virol / Year: 2009 Journal: J Virol / Year: 2009Title: Structural comparison of different antibodies interacting with parvovirus capsids. Authors: Susan Hafenstein / Valorie D Bowman / Tao Sun / Christian D S Nelson / Laura M Palermo / Paul R Chipman / Anthony J Battisti / Colin R Parrish / Michael G Rossmann /  Abstract: The structures of canine parvovirus (CPV) and feline parvovirus (FPV) complexed with antibody fragments from eight different neutralizing monoclonal antibodies were determined by cryo-electron ...The structures of canine parvovirus (CPV) and feline parvovirus (FPV) complexed with antibody fragments from eight different neutralizing monoclonal antibodies were determined by cryo-electron microscopy (cryoEM) reconstruction to resolutions varying from 8.5 to 18 A. The crystal structure of one of the Fab molecules and the sequence of the variable domain for each of the Fab molecules have been determined. The structures of Fab fragments not determined crystallographically were predicted by homology modeling according to the amino acid sequence. Fitting of the Fab and virus structures into the cryoEM densities identified the footprints of each antibody on the viral surface. As anticipated from earlier analyses, the Fab binding sites are directed to two epitopes, A and B. The A site is on an exposed part of the surface near an icosahedral threefold axis, whereas the B site is about equidistant from the surrounding five-, three-, and twofold axes. One antibody directed to the A site binds CPV but not FPV. Two of the antibodies directed to the B site neutralize the virus as Fab fragments. The differences in antibody properties have been linked to the amino acids within the antibody footprints, the position of the binding site relative to the icosahedral symmetry elements, and the orientation of the Fab structure relative to the surface of the virus. Most of the exposed surface area was antigenic, although each of the antibodies had a common area of overlap that coincided with the positions of the previously mapped escape mutations. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_5108.map.gz emd_5108.map.gz | 4.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-5108-v30.xml emd-5108-v30.xml emd-5108.xml emd-5108.xml | 10.4 KB 10.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_5108_1.png emd_5108_1.png | 289.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-5108 http://ftp.pdbj.org/pub/emdb/structures/EMD-5108 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5108 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5108 | HTTPS FTP |
-Validation report
| Summary document |  emd_5108_validation.pdf.gz emd_5108_validation.pdf.gz | 324.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_5108_full_validation.pdf.gz emd_5108_full_validation.pdf.gz | 324.5 KB | Display | |
| Data in XML |  emd_5108_validation.xml.gz emd_5108_validation.xml.gz | 5.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5108 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5108 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5108 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5108 | HTTPS FTP |
-Related structure data
| Related structure data |  3iy3MC  5105C  5106C  5107C  5109C  5110C  5111C  5112C  3gk8C  3iy0C  3iy1C  3iy2C  3iy4C  3iy5C  3iy6C  3iy7C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_5108.map.gz / Format: CCP4 / Size: 23.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_5108.map.gz / Format: CCP4 / Size: 23.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Feline panleukopenia virus in complex with FAb from neutralizing antibody MAb 8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.92 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
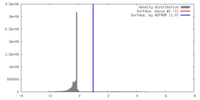
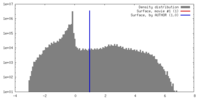
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Fab fragment from MAb 8 interacting with feline panleukopenia vir...
| Entire | Name: Fab fragment from MAb 8 interacting with feline panleukopenia virus (FPV) |
|---|---|
| Components |
|
-Supramolecule #1000: Fab fragment from MAb 8 interacting with feline panleukopenia vir...
| Supramolecule | Name: Fab fragment from MAb 8 interacting with feline panleukopenia virus (FPV) type: sample / ID: 1000 / Number unique components: 2 |
|---|
-Supramolecule #1: Feline panleukopenia virus
| Supramolecule | Name: Feline panleukopenia virus / type: virus / ID: 1 / Name.synonym: FPV / NCBI-ID: 10786 / Sci species name: Feline panleukopenia virus / Database: NCBI / Virus type: VIRION / Virus isolate: STRAIN / Virus enveloped: No / Virus empty: No / Syn species name: FPV |
|---|---|
| Host (natural) | Organism:  |
| Virus shell | Shell ID: 1 / Diameter: 280 Å / T number (triangulation number): 1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.0 mg/mL |
|---|---|
| Buffer | pH: 7.5 / Details: 10 mM Tris |
| Grid | Details: quantifoils |
| Vitrification | Cryogen name: ETHANE / Chamber temperature: 120 K / Instrument: HOMEMADE PLUNGER / Details: Vitrification instrument: plunger / Method: blot before plunging |
- Electron microscopy
Electron microscopy
| Microscope | FEI/PHILIPS CM300FEG/T |
|---|---|
| Temperature | Min: 83 K / Max: 83 K / Average: 93 K |
| Alignment procedure | Legacy - Astigmatism: lens astigmatism was corrected at 100,000 times magnification |
| Date | Jan 23, 2004 |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: ZEISS SCAI / Digitization - Sampling interval: 7 µm / Number real images: 49 / Average electron dose: 25.44 e/Å2 / Details: scanned at 7 microns and bin averaged to 14 / Od range: 0.9 / Bits/pixel: 8 |
| Electron beam | Acceleration voltage: 300 kV / Electron source: TUNGSTEN HAIRPIN |
| Electron optics | Calibrated magnification: 47190 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.0 mm / Nominal defocus max: 3.1 µm / Nominal defocus min: 1.8 µm / Nominal magnification: 45000 |
| Sample stage | Specimen holder: side mounted nitrogen cooled / Specimen holder model: GATAN LIQUID NITROGEN |
- Image processing
Image processing
| CTF correction | Details: robem |
|---|---|
| Final reconstruction | Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 11.1 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: EMPFT EM3DR / Number images used: 4344 |
 Movie
Movie Controller
Controller


 UCSF Chimera
UCSF Chimera





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)