[English] 日本語
 Yorodumi
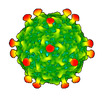
Yorodumi- EMDB-50360: Rhodobacter microvirus Ebor attached to the outer membrane vesicle -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Rhodobacter microvirus Ebor attached to the outer membrane vesicle | |||||||||||||||
 Map data Map data | Ebor virion attached to the outer membrane vesicle | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | ssDNA virus / Microviridae / Tainavirinae / Alphaproteobacteria / Rhodobacter capsulatus / aquatic virus / jelly-roll fold / VIRUS | |||||||||||||||
| Biological species |  Rhodobacter capsulatus SB 1003 (bacteria) / Rhodobacter capsulatus SB 1003 (bacteria) /  Rhodobacter phage Ebor (virus) Rhodobacter phage Ebor (virus) | |||||||||||||||
| Method | subtomogram averaging / cryo EM / Resolution: 13.2 Å | |||||||||||||||
 Authors Authors | Bardy P / Blaza JN / Jenkins HT / Nicholas TR / Konig HC / Alim NTB / Hart SJ / Turkenburg JP / Fogg PCM / Beatty JT / Antson AA | |||||||||||||||
| Funding support |  United Kingdom, United Kingdom,  Canada, 4 items Canada, 4 items
| |||||||||||||||
 Citation Citation |  Journal: bioRxiv / Year: 2024 Journal: bioRxiv / Year: 2024Title: A stargate mechanism of genome delivery unveiled by cryogenic electron tomography. Authors: Pavol Bardy / Conor I W MacDonald / Paul C Kirchberger / Huw T Jenkins / Tibor Botka / Lewis Byrom / Nawshin T B Alim / Daouda A K Traore / Hannah C König / Tristan R Nicholas / Maria ...Authors: Pavol Bardy / Conor I W MacDonald / Paul C Kirchberger / Huw T Jenkins / Tibor Botka / Lewis Byrom / Nawshin T B Alim / Daouda A K Traore / Hannah C König / Tristan R Nicholas / Maria Chechik / Samuel J Hart / Johan P Turkenburg / James N Blaza / J Thomas Beatty / Paul C M Fogg / Alfred A Antson /     Abstract: Single-stranded DNA bacteriophages of the family are major components of the global virosphere. Microviruses are highly abundant in aquatic ecosystems and are prominent members of the mammalian gut ...Single-stranded DNA bacteriophages of the family are major components of the global virosphere. Microviruses are highly abundant in aquatic ecosystems and are prominent members of the mammalian gut microbiome, where their diversity has been linked to various chronic health disorders. Despite the clear importance of microviruses, little is known about the molecular mechanism of host infection. Here, we have characterized an exceptionally large microvirus, Ebor, and provide crucial insights into long-standing mechanistic questions. Cryogenic electron microscopy of Ebor revealed a capsid with trimeric protrusions that recognise lipopolysaccharides on the host surface. Cryogenic electron tomography of the host cell colonized with virus particles demonstrated that the virus initially attaches to the cell via five such protrusions, located at the corners of a single pentamer. This interaction triggers a stargate mechanism of capsid opening along the 5-fold symmetry axis, enabling delivery of the virus genome. Despite variations in specific virus-host interactions among different family viruses, structural data indicate that the stargate mechanism of infection is universally employed by all members of the family. Startlingly, our data reveal a mechanistic link for the opening of relatively small capsids made out of a single jelly-roll fold with the structurally unrelated giant viruses. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_50360.map.gz emd_50360.map.gz | 3.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-50360-v30.xml emd-50360-v30.xml emd-50360.xml emd-50360.xml | 28.6 KB 28.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_50360_fsc.xml emd_50360_fsc.xml | 9 KB | Display |  FSC data file FSC data file |
| Images |  emd_50360.png emd_50360.png | 73.6 KB | ||
| Masks |  emd_50360_msk_1.map emd_50360_msk_1.map | 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-50360.cif.gz emd-50360.cif.gz | 6.3 KB | ||
| Others |  emd_50360_additional_1.map.gz emd_50360_additional_1.map.gz emd_50360_additional_2.map.gz emd_50360_additional_2.map.gz emd_50360_additional_3.map.gz emd_50360_additional_3.map.gz emd_50360_additional_4.map.gz emd_50360_additional_4.map.gz emd_50360_half_map_1.map.gz emd_50360_half_map_1.map.gz emd_50360_half_map_2.map.gz emd_50360_half_map_2.map.gz | 59.1 MB 59.1 MB 59.3 MB 59.3 MB 33.8 MB 33.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-50360 http://ftp.pdbj.org/pub/emdb/structures/EMD-50360 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-50360 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-50360 | HTTPS FTP |
-Validation report
| Summary document |  emd_50360_validation.pdf.gz emd_50360_validation.pdf.gz | 1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_50360_full_validation.pdf.gz emd_50360_full_validation.pdf.gz | 1 MB | Display | |
| Data in XML |  emd_50360_validation.xml.gz emd_50360_validation.xml.gz | 14.2 KB | Display | |
| Data in CIF |  emd_50360_validation.cif.gz emd_50360_validation.cif.gz | 21.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50360 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50360 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50360 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50360 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_50360.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_50360.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Ebor virion attached to the outer membrane vesicle | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 3.15 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_50360_msk_1.map emd_50360_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: strongly interacting class 2
| File | emd_50360_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | strongly interacting class 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: strongly interacting class 1
| File | emd_50360_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | strongly interacting class 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: weakly interacting class 2
| File | emd_50360_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | weakly interacting class 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: weakly interacting class 1
| File | emd_50360_additional_4.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | weakly interacting class 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_50360_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_50360_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Rhodobacter phage Ebor
| Entire | Name:  Rhodobacter phage Ebor (virus) Rhodobacter phage Ebor (virus) |
|---|---|
| Components |
|
-Supramolecule #1: Rhodobacter phage Ebor
| Supramolecule | Name: Rhodobacter phage Ebor / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all Details: purified from Rhodobacter capsulatus strain SB1003 by ion-exchange chromatography NCBI-ID: 3144506 / Sci species name: Rhodobacter phage Ebor / Virus type: VIRION / Virus isolate: OTHER / Virus enveloped: No / Virus empty: No |
|---|---|
| Host (natural) | Organism:  Rhodobacter capsulatus (bacteria) Rhodobacter capsulatus (bacteria) |
| Molecular weight | Theoretical: 5.7 MDa |
| Virus shell | Shell ID: 1 / Name: Native capsid / Diameter: 460.0 Å / T number (triangulation number): 1 |
-Macromolecule #1: Major capsid protein
| Macromolecule | Name: Major capsid protein / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Rhodobacter capsulatus SB 1003 (bacteria) / Strain: SB1003 Rhodobacter capsulatus SB 1003 (bacteria) / Strain: SB1003 |
| Sequence | String: MSYQTKRNVR REARTLMGRF KAGKLAPVMA VPVKGSEGGM LSQSVSFELD PIAGRMATPI TAEMCAVFVP VQACDALKNP EADYAGMTEI VREKLLSGNP LFVLEPETDV SKRCGVNPRR NNGLMRVNEI VRLAHNCAVN FLRRRRYVDA VQLTAANHST TPAILSQTVL ...String: MSYQTKRNVR REARTLMGRF KAGKLAPVMA VPVKGSEGGM LSQSVSFELD PIAGRMATPI TAEMCAVFVP VQACDALKNP EADYAGMTEI VREKLLSGNP LFVLEPETDV SKRCGVNPRR NNGLMRVNEI VRLAHNCAVN FLRRRRYVDA VQLTAANHST TPAILSQTVL DRFNGALDPD PNVNGAVQLS MPDMRLPVAS DAEKAVTAPL TVKRGNENRR LDAGISRLAA AEVAAATDAA LYAVFGGREA GNVSLTDFYN AQKMDELTRV MRKICDDNPE YGEEMVLRWA HGLSVDPGRV PFLLAEKSVV LGRQIIGATD TAGVEDGVKR SDMAAQLSFT VPIPTTELGG IIVTFACIKP DETLSSQPHP ILADHWRLDN FVADELALDP QPVMARELDY KVAQANETTV VFYTGLNELK KTYVSYGLCR ALDPNTVESK NAVWQLEVPL SVTPETVLYP ADLPQYPFAD QQAEVCTYVV QSTAVMPTPM IFGPSPVEQL AVIETEDLFE D |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 / Component - Concentration: 10.0 mM / Component - Name: Tris-HCl |
|---|---|
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: NITROGEN / Chamber humidity: 90 % / Instrument: FEI VITROBOT MARK IV Details: single side blotting, 5.5 sec blotting time, 2 sec wait time, 0 force, 0 drain time. |
| Details | phage Ebor mixed with outer membrane vesicles in titre of 7x10^10 PFU/ml |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Number real images: 1 / Average electron dose: 1.9 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 45000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)













































































