+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of DIM2-HP1-H3K9me3-DNA complex | |||||||||
 Map data Map data | sharpened map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | DNA methyltransferase / Transferase-DNA Binding Protein-DNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationorganic cyclic compound binding / DNA (cytosine-5-)-methyltransferase / DNA (cytosine-5-)-methyltransferase activity / pericentric heterochromatin / methylated histone binding / structural constituent of chromatin / nucleosome / methylation / chromatin remodeling / protein heterodimerization activity ...organic cyclic compound binding / DNA (cytosine-5-)-methyltransferase / DNA (cytosine-5-)-methyltransferase activity / pericentric heterochromatin / methylated histone binding / structural constituent of chromatin / nucleosome / methylation / chromatin remodeling / protein heterodimerization activity / chromatin binding / negative regulation of transcription by RNA polymerase II / DNA binding / nucleus Similarity search - Function | |||||||||
| Biological species |  Neurospora crassa (fungus) Neurospora crassa (fungus) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.79 Å | |||||||||
 Authors Authors | Song J / Shao Z | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: CryoEM structure of DIM2-HP1-H3K9me3-DNA complex Authors: Song J / Shao Z | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_44411.map.gz emd_44411.map.gz | 59.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-44411-v30.xml emd-44411-v30.xml emd-44411.xml emd-44411.xml | 20.3 KB 20.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_44411.png emd_44411.png | 67.1 KB | ||
| Filedesc metadata |  emd-44411.cif.gz emd-44411.cif.gz | 6.8 KB | ||
| Others |  emd_44411_additional_1.map.gz emd_44411_additional_1.map.gz emd_44411_half_map_1.map.gz emd_44411_half_map_1.map.gz emd_44411_half_map_2.map.gz emd_44411_half_map_2.map.gz | 32.4 MB 59.5 MB 59.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-44411 http://ftp.pdbj.org/pub/emdb/structures/EMD-44411 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44411 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44411 | HTTPS FTP |
-Validation report
| Summary document |  emd_44411_validation.pdf.gz emd_44411_validation.pdf.gz | 876.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_44411_full_validation.pdf.gz emd_44411_full_validation.pdf.gz | 875.8 KB | Display | |
| Data in XML |  emd_44411_validation.xml.gz emd_44411_validation.xml.gz | 12.3 KB | Display | |
| Data in CIF |  emd_44411_validation.cif.gz emd_44411_validation.cif.gz | 14.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44411 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44411 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44411 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44411 | HTTPS FTP |
-Related structure data
| Related structure data |  9baqMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_44411.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_44411.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | sharpened map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.056 Å | ||||||||||||||||||||||||||||||||||||
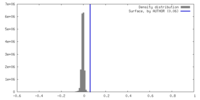
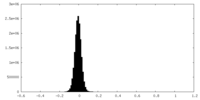
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: unsharpened map
| File | emd_44411_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | unsharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
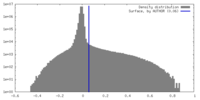
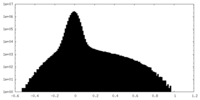
| Density Histograms |
-Half map: Half map A
| File | emd_44411_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
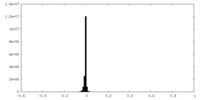
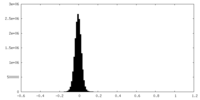
| Density Histograms |
-Half map: Half map B
| File | emd_44411_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
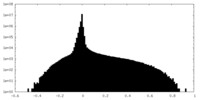
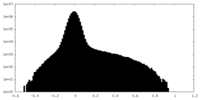
| Density Histograms |
- Sample components
Sample components
-Entire : DIM2-HP1-H3K9m3-DNA
| Entire | Name: DIM2-HP1-H3K9m3-DNA |
|---|---|
| Components |
|
-Supramolecule #1: DIM2-HP1-H3K9m3-DNA
| Supramolecule | Name: DIM2-HP1-H3K9m3-DNA / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 |
|---|---|
| Source (natural) | Organism:  Neurospora crassa (fungus) Neurospora crassa (fungus) |
-Macromolecule #1: DNA (cytosine-5-)-methyltransferase
| Macromolecule | Name: DNA (cytosine-5-)-methyltransferase / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: DNA (cytosine-5-)-methyltransferase |
|---|---|
| Source (natural) | Organism:  Neurospora crassa (fungus) Neurospora crassa (fungus) |
| Molecular weight | Theoretical: 139.935891 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GSMDSPDRSH GGMFIDVPAE TMGFQEDYLD MFASVLSQGL AKEGDYAHHQ PLPAGKEECL EPIAVATTIT PSPDDPQLQL QLELEQQFQ TESGLNGVDP APAPESEDEA DLPDGFSDES PDDDFVVQRS KHITVDLPVS TLINPRSTFQ RIDENDNLVP P PQSTPERV ...String: GSMDSPDRSH GGMFIDVPAE TMGFQEDYLD MFASVLSQGL AKEGDYAHHQ PLPAGKEECL EPIAVATTIT PSPDDPQLQL QLELEQQFQ TESGLNGVDP APAPESEDEA DLPDGFSDES PDDDFVVQRS KHITVDLPVS TLINPRSTFQ RIDENDNLVP P PQSTPERV AVEDLLKAAK AAGKNKEDYI EFELHDFNFY VNYAYHPQEM RPIQLVATKV LHDKYYFDGV LKYGNTKHYV TG MQVLELP VGNYGASLHS VKGQIWVRSK HNAKKEIYYL LKKPAFEYQR YYQPFLWIAD LGKHVVDYCT RMVERKREVT LGC FKSDFI QWASKAHGKS KAFQNWRAQH PSDDFRTSVA ANIGYIWKEI NGVAGAKRAA GDQLFRELMI VKPGQYFRQE VPPG PVVTE GDRTVAATIV TPYIKECFGH MILGKVLRLA GEDAEKEKEV KLAKRLKIEN KNATKADTKD DMKNDTATES LPTPL RSLP VQVLEATPIE SDIVSIVSSD LPPSENNPPP LTNGSVKPKA KANPKPKPST QPLHAAHVKY LSQELVNKIK VGDVIS TPR DDSSNTDTKW KPTDTDDHRW FGLVQRVHTA KTKSSGRGLN SKSFDVIWFY RPEDTPCCAM KYKWRNELFL SNHCTCQ EG HHARVKGNEV LAVHPVDWFG TPESNKGEFF VRQLYESEQR RWITLQKDHL TCYHNQPPKP PTAPYKPGDT VLATLSPS D KFSDPYEVVE YFTQGEKETA FVRLRKLLRR RKVDRQDAPA NELVYTEDLV DVRAERIVGK CIMRCFRPDE RVPSPYDRG GTGNMFFITH RQDHGRCVPL DTLPPTLRQG FNPLGNLGKP KLRGMDLYCG GGNFGRGLEE GGVVEMRWAN DIWDKAIHTY MANTPDPNK TNPFLGSVDD LLRLALEGKF SDNVPRPGEV DFIAAGSPCP GFSLLTQDKK VLNQVKNQSL VASFASFVDF Y RPKYGVLE NVSGIVQTFV NRKQDVLSQL FCALVGMGYQ AQLILGDAWA HGAPQSRERV FLYFAAPGLP LPDPPLPSHS HY RVKNRNI GFLCNGESYV QRSFIPTAFK FVSAGEGTAD LPKIGDGKPD ACVRFPDHRL ASGITPYIRA QYACIPTHPY GMN FIKAWN NGNGVMSKSD RDLFPSEGKT RTSDASVGWK RLNPKTLFPT VTTTSNPSDA RMGPGLHWDE DRPYTVQEMR RAQG YLDEE VLVGRTTDQW KLVGNSVSRH MALAIGLKFR EAWLGTLYD UniProtKB: DNA (cytosine-5-)-methyltransferase |
-Macromolecule #2: Heterochromatin protein one
| Macromolecule | Name: Heterochromatin protein one / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Neurospora crassa (fungus) Neurospora crassa (fungus) |
| Molecular weight | Theoretical: 30.489086 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GSMPYDPSAL SDEEAASSVE LDTRSATSSS KKQSRDKKSV KYTIPEPEDF EDEEQNGDGA DEGGEDDEEG DEEEEDVYVV EKILDHMLN DDNEPLFLVK WEGYEKKSDQ TWEPEDTLIE GASERLKEYF TKIGGREKIF EASAAAQKIK KRGRPSSNSG T PQASSNKR ...String: GSMPYDPSAL SDEEAASSVE LDTRSATSSS KKQSRDKKSV KYTIPEPEDF EDEEQNGDGA DEGGEDDEEG DEEEEDVYVV EKILDHMLN DDNEPLFLVK WEGYEKKSDQ TWEPEDTLIE GASERLKEYF TKIGGREKIF EASAAAQKIK KRGRPSSNSG T PQASSNKR SRKNGDHPLN SEEPQTAKNA AWKPPAGSWE EHIAQLDACE DEDTHKLMVY LTWKNGHKTQ HTTDVIYKRC PQ KMLQFYE RHVRIIKRDP DSEDREGSVS Q UniProtKB: Heterochromatin protein one |
-Macromolecule #3: Histone H3.2
| Macromolecule | Name: Histone H3.2 / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Neurospora crassa (fungus) Neurospora crassa (fungus) |
| Molecular weight | Theoretical: 2.791302 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: ARTKQTAR(M3L)S TGGKAPRKQL ATKAW UniProtKB: Histone H3.2 |
-Macromolecule #4: DNA (5'-D(*AP*GP*TP*AP*GP*GP*AP*GP*GP*AP*GP*GP*AP*GP*TP*AP*GP*T)-3')
| Macromolecule | Name: DNA (5'-D(*AP*GP*TP*AP*GP*GP*AP*GP*GP*AP*GP*GP*AP*GP*TP*AP*GP*T)-3') type: dna / ID: 4 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Neurospora crassa (fungus) Neurospora crassa (fungus) |
| Molecular weight | Theoretical: 5.709714 KDa |
| Sequence | String: (DA)(DG)(DT)(DA)(DG)(DG)(DA)(DG)(DG)(DA) (DG)(DG)(DA)(DG)(DT)(DA)(DG)(DT) |
-Macromolecule #5: DNA (5'-D(*AP*CP*TP*AP*CP*T)-R(P*(PYO))-D(P*CP*TP*CP*CP*TP*CP*CP*...
| Macromolecule | Name: DNA (5'-D(*AP*CP*TP*AP*CP*T)-R(P*(PYO))-D(P*CP*TP*CP*CP*TP*CP*CP*TP*AP*CP*T)-3') type: dna / ID: 5 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Neurospora crassa (fungus) Neurospora crassa (fungus) |
| Molecular weight | Theoretical: 5.323441 KDa |
| Sequence | String: (DA)(DC)(DT)(DA)(DC)(DT)(PYO)(DC)(DT)(DC) (DC)(DT)(DC)(DC)(DT)(DA)(DC)(DT) |
-Macromolecule #6: S-ADENOSYL-L-HOMOCYSTEINE
| Macromolecule | Name: S-ADENOSYL-L-HOMOCYSTEINE / type: ligand / ID: 6 / Number of copies: 1 / Formula: SAH |
|---|---|
| Molecular weight | Theoretical: 384.411 Da |
| Chemical component information |  ChemComp-SAH: |
-Macromolecule #7: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 7 / Number of copies: 1 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.79 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 128667 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
 Movie
Movie Controller
Controller







 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)