+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM Structure of Diffocin - precontracted - Collar | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Phage tail-like / bacteriocin / collar / pre-contraction / VIRUS LIKE PARTICLE | ||||||||||||
| Function / homology |  Function and homology information Function and homology information: / Domain of unknown function (DUF6838) / Phage tail tube protein / XkdM-like superfamily / Phage tail tube protein / Tail sheath protein, subtilisin-like domain / Phage tail sheath protein subtilisin-like domain / Tail sheath protein, C-terminal domain / Phage tail sheath C-terminal domain Similarity search - Domain/homology | ||||||||||||
| Biological species |  Clostridioides difficile (bacteria) Clostridioides difficile (bacteria) | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.7 Å | ||||||||||||
 Authors Authors | Cai XY / He Y / Zhou ZH | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Atomic structures of a bacteriocin targeting Gram-positive bacteria. Authors: Xiaoying Cai / Yao He / Iris Yu / Anthony Imani / Dean Scholl / Jeff F Miller / Z Hong Zhou /  Abstract: Due to envelope differences between Gram-positive and Gram-negative bacteria, engineering precision bactericidal contractile nanomachines requires atomic-level understanding of their structures; ...Due to envelope differences between Gram-positive and Gram-negative bacteria, engineering precision bactericidal contractile nanomachines requires atomic-level understanding of their structures; however, only those killing Gram-negative bacteria are currently known. Here, we report the atomic structures of an engineered diffocin, a contractile syringe-like molecular machine that kills the Gram-positive bacterium Clostridioides difficile. Captured in one pre-contraction and two post-contraction states, each structure fashions six proteins in the bacteria-targeting baseplate, two proteins in the energy-storing trunk, and a collar linking the sheath with the membrane-penetrating tube. Compared to contractile machines targeting Gram-negative bacteria, major differences reside in the baseplate and contraction magnitude, consistent with target envelope differences. The multifunctional hub-hydrolase protein connects the tube and baseplate and is positioned to degrade peptidoglycan during penetration. The full-length tape measure protein forms a coiled-coil helix bundle homotrimer spanning the entire diffocin. Our study offers mechanical insights and principles for designing potent protein-based precision antibiotics. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_42953.map.gz emd_42953.map.gz | 96.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-42953-v30.xml emd-42953-v30.xml emd-42953.xml emd-42953.xml | 16.6 KB 16.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_42953.png emd_42953.png | 63.7 KB | ||
| Masks |  emd_42953_msk_1.map emd_42953_msk_1.map | 103 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-42953.cif.gz emd-42953.cif.gz | 5.9 KB | ||
| Others |  emd_42953_half_map_1.map.gz emd_42953_half_map_1.map.gz emd_42953_half_map_2.map.gz emd_42953_half_map_2.map.gz | 79 MB 79 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-42953 http://ftp.pdbj.org/pub/emdb/structures/EMD-42953 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42953 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42953 | HTTPS FTP |
-Validation report
| Summary document |  emd_42953_validation.pdf.gz emd_42953_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_42953_full_validation.pdf.gz emd_42953_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_42953_validation.xml.gz emd_42953_validation.xml.gz | 12.8 KB | Display | |
| Data in CIF |  emd_42953_validation.cif.gz emd_42953_validation.cif.gz | 15 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42953 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42953 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42953 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42953 | HTTPS FTP |
-Related structure data
| Related structure data |  8v3tMC  8v3wC  8v3xC  8v3yC  8v3zC  8v40C  8v41C  8v43C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_42953.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_42953.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.1 Å | ||||||||||||||||||||||||||||||||||||
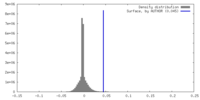
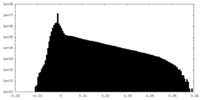
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_42953_msk_1.map emd_42953_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
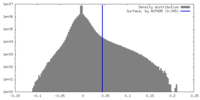
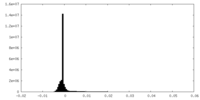
| Density Histograms |
-Half map: #2
| File | emd_42953_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
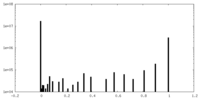
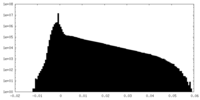
| Density Histograms |
-Half map: #1
| File | emd_42953_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
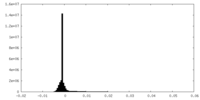
| Density Histograms |
- Sample components
Sample components
-Entire : Diffocin
| Entire | Name: Diffocin |
|---|---|
| Components |
|
-Supramolecule #1: Diffocin
| Supramolecule | Name: Diffocin / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Clostridioides difficile (bacteria) Clostridioides difficile (bacteria) |
-Macromolecule #1: Sheath (CD1363)
| Macromolecule | Name: Sheath (CD1363) / type: protein_or_peptide / ID: 1 / Number of copies: 18 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Clostridioides difficile (bacteria) Clostridioides difficile (bacteria) |
| Molecular weight | Theoretical: 39.26843 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAIGLPSINI SFKELATTVK ERSARGIIAM VLKDAKALGL NEIHEKEDIP VDLSAENKEY INLALMGNVN TPNKLLVYVI EGEADIQTA LDFLETKEFN YLCMPKAVEA DKTAIKNWII KLRDIDKVKV KAVLGKVVGN HEGIINFTTE DVLVGEKKYS V DEFTSRVA ...String: MAIGLPSINI SFKELATTVK ERSARGIIAM VLKDAKALGL NEIHEKEDIP VDLSAENKEY INLALMGNVN TPNKLLVYVI EGEADIQTA LDFLETKEFN YLCMPKAVEA DKTAIKNWII KLRDIDKVKV KAVLGKVVGN HEGIINFTTE DVLVGEKKYS V DEFTSRVA GLIAGTPLSQ SVTYTKLSDV VDIPKMTKVD AESRVNKGEL ILIKEAGAIR IARGVNSLTE LTAEKGEMFQ KI KIVDTLD IIHSDIRKVI IDDYIGKVTN SYDNKCLLIV AIKSYLEELE KSALIESDST VEIDFEAQKS YLKSKGVDLS YMT LQEIKE ANTGSKVFLK AKIKVLDAME DIDLSIEI UniProtKB: Phage tail sheath protein |
-Macromolecule #2: Tube (CD1364)
| Macromolecule | Name: Tube (CD1364) / type: protein_or_peptide / ID: 2 / Number of copies: 18 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Clostridioides difficile (bacteria) Clostridioides difficile (bacteria) |
| Molecular weight | Theoretical: 16.028353 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MANMEARNVM SGTWGELWLD GNKVAEVKKF QAKMEFTKED IIIAGQMGTD TKYMGYKGKG SITLYHVSSR MHKLIGEKIK RGSEPRFVA ISKLNDPDSY GAERIAVKNI AFDDLTLADW EVGVKGEIEA PFTFTEYDFL DII UniProtKB: Phage portal protein |
-Macromolecule #3: Collar (CD1362)
| Macromolecule | Name: Collar (CD1362) / type: protein_or_peptide / ID: 3 / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Clostridioides difficile (bacteria) Clostridioides difficile (bacteria) |
| Molecular weight | Theoretical: 17.198816 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MLKYKEILET IIEILKKNFT ESIFIDDESV QGSEGSCFFV SILSVICTPV MLNTNNKDIV ISIKYLPKPQ SKSIRMYEIS DELNKLFNR NIKVTDRKLN ITKLEQSIKK EESIYVLNFT FTLNYLDSVY EEDVVYENMK EINLNLGE UniProtKB: RtbA |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.7 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 144368 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)