[English] 日本語
 Yorodumi
Yorodumi- EMDB-42676: 5-HT2AR bound to Lisuride in complex with a mini-Gq protein and a... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | 5-HT2AR bound to Lisuride in complex with a mini-Gq protein and an active-state stabilizing single-chain variable fragment (scFv16) obtained by cryo-electron microscopy (cryoEM) | |||||||||
 Map data Map data | Composite map for 5-HT2AR bound to lisuride in complex with a mini-Gq protein and an active-state stabilizing single-chain variable fragment (scFv16) obtained by cryo-electron microscopy | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | 5-HT2A receptor / serotonin receptor / G protein / GPCR / Lisuride / cryoEM / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of heat generation / protein localization to cytoskeleton / 1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine binding / Gq/11-coupled serotonin receptor activity / phospholipase C-activating serotonin receptor signaling pathway / positive regulation of phosphatidylinositol biosynthetic process / G protein-coupled serotonin receptor signaling pathway / G protein-coupled serotonin receptor complex / neurofilament / sensitization ...positive regulation of heat generation / protein localization to cytoskeleton / 1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine binding / Gq/11-coupled serotonin receptor activity / phospholipase C-activating serotonin receptor signaling pathway / positive regulation of phosphatidylinositol biosynthetic process / G protein-coupled serotonin receptor signaling pathway / G protein-coupled serotonin receptor complex / neurofilament / sensitization / positive regulation of cytokine production involved in immune response / Serotonin receptors / cell body fiber / artery smooth muscle contraction / serotonin receptor signaling pathway / urinary bladder smooth muscle contraction / serotonin binding / negative regulation of synaptic transmission, glutamatergic / G protein-coupled serotonin receptor activity / positive regulation of platelet aggregation / neurotransmitter receptor activity / positive regulation of DNA biosynthetic process / temperature homeostasis / protein tyrosine kinase activator activity / regulation of dopamine secretion / negative regulation of potassium ion transport / positive regulation of execution phase of apoptosis / detection of temperature stimulus involved in sensory perception of pain / behavioral response to cocaine / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / positive regulation of fat cell differentiation / detection of mechanical stimulus involved in sensory perception of pain / positive regulation of vasoconstriction / release of sequestered calcium ion into cytosol / presynaptic modulation of chemical synaptic transmission / phosphatidylinositol 3-kinase/protein kinase B signal transduction / positive regulation of glycolytic process / dendritic shaft / glycolytic process / Olfactory Signaling Pathway / caveola / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / G-protein activation / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / memory / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through CDC42 / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / photoreceptor disc membrane / positive regulation of inflammatory response / Glucagon-type ligand receptors / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / intracellular calcium ion homeostasis / G alpha (z) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / ADORA2B mediated anti-inflammatory cytokines production / cellular response to catecholamine stimulus / sensory perception of taste / positive regulation of neuron apoptotic process / ADP signalling through P2Y purinoceptor 1 / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / positive regulation of peptidyl-tyrosine phosphorylation / GPER1 signaling / cellular response to prostaglandin E stimulus / G-protein beta-subunit binding / Inactivation, recovery and regulation of the phototransduction cascade / heterotrimeric G-protein complex / G alpha (12/13) signalling events / extracellular vesicle / signaling receptor complex adaptor activity / Thrombin signalling through proteinase activated receptors (PARs) / GTPase binding / retina development in camera-type eye / virus receptor activity / presynaptic membrane / Ca2+ pathway / phospholipase C-activating G protein-coupled receptor signaling pathway / positive regulation of cytosolic calcium ion concentration / G alpha (i) signalling events / fibroblast proliferation / cytoplasmic vesicle / G alpha (s) signalling events / chemical synaptic transmission / G alpha (q) signalling events / postsynaptic membrane / Ras protein signal transduction / cell population proliferation / Extra-nuclear estrogen signaling Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.8 Å | |||||||||
 Authors Authors | Barros-Alvarez X / Kim K / Panova O / Roth BL / Skiniotis G | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2024 Journal: Science / Year: 2024Title: AlphaFold2 structures guide prospective ligand discovery. Authors: Jiankun Lyu / Nicholas Kapolka / Ryan Gumpper / Assaf Alon / Liang Wang / Manish K Jain / Ximena Barros-Álvarez / Kensuke Sakamoto / Yoojoong Kim / Jeffrey DiBerto / Kuglae Kim / Isabella S ...Authors: Jiankun Lyu / Nicholas Kapolka / Ryan Gumpper / Assaf Alon / Liang Wang / Manish K Jain / Ximena Barros-Álvarez / Kensuke Sakamoto / Yoojoong Kim / Jeffrey DiBerto / Kuglae Kim / Isabella S Glenn / Tia A Tummino / Sijie Huang / John J Irwin / Olga O Tarkhanova / Yurii Moroz / Georgios Skiniotis / Andrew C Kruse / Brian K Shoichet / Bryan L Roth /   Abstract: AlphaFold2 (AF2) models have had wide impact but mixed success in retrospective ligand recognition. We prospectively docked large libraries against unrefined AF2 models of the σ and serotonin 2A (5- ...AlphaFold2 (AF2) models have had wide impact but mixed success in retrospective ligand recognition. We prospectively docked large libraries against unrefined AF2 models of the σ and serotonin 2A (5-HT2A) receptors, testing hundreds of new molecules and comparing results with those obtained from docking against the experimental structures. Hit rates were high and similar for the experimental and AF2 structures, as were affinities. Success in docking against the AF2 models was achieved despite differences between orthosteric residue conformations in the AF2 models and the experimental structures. Determination of the cryo-electron microscopy structure for one of the more potent 5-HT2A ligands from the AF2 docking revealed residue accommodations that resembled the AF2 prediction. AF2 models may sample conformations that differ from experimental structures but remain low energy and relevant for ligand discovery, extending the domain of structure-based drug design. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_42676.map.gz emd_42676.map.gz | 1.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-42676-v30.xml emd-42676-v30.xml emd-42676.xml emd-42676.xml | 25 KB 25 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_42676.png emd_42676.png | 53.2 KB | ||
| Filedesc metadata |  emd-42676.cif.gz emd-42676.cif.gz | 7.2 KB | ||
| Others |  emd_42676_additional_1.map.gz emd_42676_additional_1.map.gz emd_42676_additional_2.map.gz emd_42676_additional_2.map.gz emd_42676_additional_3.map.gz emd_42676_additional_3.map.gz | 815 KB 1.8 MB 89.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-42676 http://ftp.pdbj.org/pub/emdb/structures/EMD-42676 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42676 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42676 | HTTPS FTP |
-Validation report
| Summary document |  emd_42676_validation.pdf.gz emd_42676_validation.pdf.gz | 346 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_42676_full_validation.pdf.gz emd_42676_full_validation.pdf.gz | 345.5 KB | Display | |
| Data in XML |  emd_42676_validation.xml.gz emd_42676_validation.xml.gz | 6.8 KB | Display | |
| Data in CIF |  emd_42676_validation.cif.gz emd_42676_validation.cif.gz | 7.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42676 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42676 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42676 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42676 | HTTPS FTP |
-Related structure data
| Related structure data |  8uwlMC  8v6uC  43630  43631 M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_42676.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_42676.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Composite map for 5-HT2AR bound to lisuride in complex with a mini-Gq protein and an active-state stabilizing single-chain variable fragment (scFv16) obtained by cryo-electron microscopy | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.8521 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Local map for 5-HT2AR bound to Lisuride
| File | emd_42676_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local map for 5-HT2AR bound to Lisuride | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Local map for mini-Gq protein and an active-state...
| File | emd_42676_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local map for mini-Gq protein and an active-state stabilizing single-chain variable fragment (scFv16) obtained by cryo-electron microscopy | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Global refinement map previous to local refinement
| File | emd_42676_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Global refinement map previous to local refinement | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : 5-HT2AR bound to Lisuride in complex with heterotrimeric mini-Gq ...
| Entire | Name: 5-HT2AR bound to Lisuride in complex with heterotrimeric mini-Gq protein and single-chain variable fragment (scFv16) |
|---|---|
| Components |
|
-Supramolecule #1: 5-HT2AR bound to Lisuride in complex with heterotrimeric mini-Gq ...
| Supramolecule | Name: 5-HT2AR bound to Lisuride in complex with heterotrimeric mini-Gq protein and single-chain variable fragment (scFv16) type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: 5-hydroxytryptamine receptor 2A
| Macromolecule | Name: 5-hydroxytryptamine receptor 2A / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 38.274285 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: LSLLHLQEKN WSALLTAVVI ILTIAGNILV IMAVSLEKKL QNATNYFLMS LAIADMLLGF LVMPVSMLTI LYGYRWPLPS KLCAVWIYL DVLFSTASIM HLCAISLDRY VAIQNPIHHS RFNSRTKAFL KIIAVWTISV GISMPIPVFG LQDDSKVFKE G SCLLADDN ...String: LSLLHLQEKN WSALLTAVVI ILTIAGNILV IMAVSLEKKL QNATNYFLMS LAIADMLLGF LVMPVSMLTI LYGYRWPLPS KLCAVWIYL DVLFSTASIM HLCAISLDRY VAIQNPIHHS RFNSRTKAFL KIIAVWTISV GISMPIPVFG LQDDSKVFKE G SCLLADDN FVLIGSFVSF FIPLTIMVIT YFLTIKSLQK EATLCVSDLG TRAKLASFSF LPQSSLSSEK LFQRSIHREP GS YTGRRTM QSISNEQKAC KVLGIVFFLF VVMWCPFFIT NIMAVICKES CNEDVIGALL NVFVWIGYLS SAVNPLVYTL FNK TYRSAF SRYIQCQYKE NKK UniProtKB: 5-hydroxytryptamine receptor 2A |
-Macromolecule #2: G protein subunit q (Gi2-mini-Gq chimera)
| Macromolecule | Name: G protein subunit q (Gi2-mini-Gq chimera) / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 28.084832 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGSTVSAEDK AAAERSKMID KNLREDGEKA RRTLRLLLLG ADNSGKSTIV KQMRILHGGS GGSGGTSGIF ETKFQVDKVN FHMFDVGGQ RDERRKWIQC FNDVTAIIFV VDSSDYNRLQ EALNDFKSIW NNRWLRTISV ILFLNKQDLL AEKVLAGKSK I EDYFPEFA ...String: MGSTVSAEDK AAAERSKMID KNLREDGEKA RRTLRLLLLG ADNSGKSTIV KQMRILHGGS GGSGGTSGIF ETKFQVDKVN FHMFDVGGQ RDERRKWIQC FNDVTAIIFV VDSSDYNRLQ EALNDFKSIW NNRWLRTISV ILFLNKQDLL AEKVLAGKSK I EDYFPEFA RYTTPEDATP EPGEDPRVTR AKYFIRKEFV DISTASGDGR HICYPHFTCA VDTENARRIF NDCKDIILQM NL REYNLV |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 37.41693 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSELDQLRQE AEQLKNQIRD ARKACADATL SQITNNIDPV GRIQMRTRRT LRGHLAKIYA MHWGTDSRLL VSASQDGKLI IWDSYTTNK VHAIPLRSSW VMTCAYAPSG NYVACGGLDN ICSIYNLKTR EGNVRVSREL AGHTGYLSCC RFLDDNQIVT S SGDTTCAL ...String: MSELDQLRQE AEQLKNQIRD ARKACADATL SQITNNIDPV GRIQMRTRRT LRGHLAKIYA MHWGTDSRLL VSASQDGKLI IWDSYTTNK VHAIPLRSSW VMTCAYAPSG NYVACGGLDN ICSIYNLKTR EGNVRVSREL AGHTGYLSCC RFLDDNQIVT S SGDTTCAL WDIETGQQTT TFTGHTGDVM SLSLAPDTRL FVSGACDASA KLWDVREGMC RQTFTGHESD INAICFFPNG NA FATGSDD ATCRLFDLRA DQELMTYSHD NIICGITSVS FSKSGRLLLA GYDDFNCNVW DALKADRAGV LAGHDNRVSC LGV TDDGMA VATGSWDSFL KIWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #5: Single-chain variable fragment 16 (scFv16)
| Macromolecule | Name: Single-chain variable fragment 16 (scFv16) / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 30.552234 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MLLVNQSHQG FNKEHTSKMV SAIVLYVLLA AAAHSAFADV QLVESGGGLV QPGGSRKLSC SASGFAFSSF GMHWVRQAPE KGLEWVAYI SSGSGTIYYA DTVKGRFTIS RDDPKNTLFL QMTSLRSEDT AMYYCVRSIY YYGSSPFDFW GQGTTLTVSS G GGGSGGGG ...String: MLLVNQSHQG FNKEHTSKMV SAIVLYVLLA AAAHSAFADV QLVESGGGLV QPGGSRKLSC SASGFAFSSF GMHWVRQAPE KGLEWVAYI SSGSGTIYYA DTVKGRFTIS RDDPKNTLFL QMTSLRSEDT AMYYCVRSIY YYGSSPFDFW GQGTTLTVSS G GGGSGGGG SGGGGSDIVM TQATSSVPVT PGESVSISCR SSKSLLHSNG NTYLYWFLQR PGQSPQLLIY RMSNLASGVP DR FSGSGSG TAFTLTISRL EAEDVGVYYC MQHLEYPLTF GAGTKLELK |
-Macromolecule #6: N,N-diethyl-N'-[(8alpha)-6-methyl-9,10-didehydroergolin-8-yl]urea
| Macromolecule | Name: N,N-diethyl-N'-[(8alpha)-6-methyl-9,10-didehydroergolin-8-yl]urea type: ligand / ID: 6 / Number of copies: 1 / Formula: H8G |
|---|---|
| Molecular weight | Theoretical: 338.447 Da |
| Chemical component information | 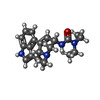 ChemComp-H8G: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 15 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: UltrAuFoil R1.2/1.3 |
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 67.78 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Calibrated magnification: 57050 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.8 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 2.8 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC / Number images used: 133216 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION / Software - Name: cryoSPARC |
| Final angle assignment | Type: ANGULAR RECONSTITUTION / Software - Name: cryoSPARC |
 Movie
Movie Controller
Controller

























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)