[English] 日本語
 Yorodumi
Yorodumi- EMDB-42511: Structural and biochemical investigations of a HEAT-repeat protei... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structural and biochemical investigations of a HEAT-repeat protein involved in the cytosolic iron-sulfur cluster assembly pathway | |||||||||||||||
 Map data Map data | Primary Map (z-flipped) | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | IRON-SULFUR CLUSTER / METALLOCOFACTOR / ASSEMBLY / METAL TRANSPORT | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationcytosolic [4Fe-4S] assembly targeting complex / protein maturation by iron-sulfur cluster transfer / DNA metabolic process / iron-sulfur cluster assembly / response to UV / DNA repair / nucleus / cytosol / cytoplasm Similarity search - Function | |||||||||||||||
| Biological species |  | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 12.77 Å | |||||||||||||||
 Authors Authors | Vasquez S / Drennan CL | |||||||||||||||
| Funding support |  United States, 4 items United States, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Commun Biol / Year: 2023 Journal: Commun Biol / Year: 2023Title: Structural and biochemical investigations of a HEAT-repeat protein involved in the cytosolic iron-sulfur cluster assembly pathway. Authors: Sheena Vasquez / Melissa D Marquez / Edward J Brignole / Amanda Vo / Sunnie Kong / Christopher Park / Deborah L Perlstein / Catherine L Drennan /  Abstract: Iron-sulfur clusters are essential for life and defects in their biosynthesis lead to human diseases. The mechanism of cluster assembly and delivery to cytosolic and nuclear client proteins via the ...Iron-sulfur clusters are essential for life and defects in their biosynthesis lead to human diseases. The mechanism of cluster assembly and delivery to cytosolic and nuclear client proteins via the cytosolic iron-sulfur cluster assembly (CIA) pathway is not well understood. Here we report cryo-EM structures of the HEAT-repeat protein Met18 from Saccharomyces cerevisiae, a key component of the CIA targeting complex (CTC) that identifies cytosolic and nuclear client proteins and delivers a mature iron-sulfur cluster. We find that in the absence of other CTC proteins, Met18 adopts tetrameric and hexameric states. Using mass photometry and negative stain EM, we show that upon the addition of Cia2, these higher order oligomeric states of Met18 disassemble. We also use pulldown assays to identify residues of critical importance for Cia2 binding and recognition of the Leu1 client, many of which are buried when Met18 oligomerizes. Our structures show conformations of Met18 that have not been previously observed in any Met18 homolog, lending support to the idea that a highly flexible Met18 may be key to how the CTC is able to deliver iron-sulfur clusters to client proteins of various sizes and shapes, i.e. Met18 conforms to the dimensions needed. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_42511.map.gz emd_42511.map.gz | 70.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-42511-v30.xml emd-42511-v30.xml emd-42511.xml emd-42511.xml | 19 KB 19 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_42511.png emd_42511.png | 64.7 KB | ||
| Filedesc metadata |  emd-42511.cif.gz emd-42511.cif.gz | 6.7 KB | ||
| Others |  emd_42511_half_map_1.map.gz emd_42511_half_map_1.map.gz emd_42511_half_map_2.map.gz emd_42511_half_map_2.map.gz | 69.8 MB 69.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-42511 http://ftp.pdbj.org/pub/emdb/structures/EMD-42511 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42511 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42511 | HTTPS FTP |
-Validation report
| Summary document |  emd_42511_validation.pdf.gz emd_42511_validation.pdf.gz | 891.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_42511_full_validation.pdf.gz emd_42511_full_validation.pdf.gz | 890.6 KB | Display | |
| Data in XML |  emd_42511_validation.xml.gz emd_42511_validation.xml.gz | 12.8 KB | Display | |
| Data in CIF |  emd_42511_validation.cif.gz emd_42511_validation.cif.gz | 15.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42511 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42511 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42511 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42511 | HTTPS FTP |
-Related structure data
| Related structure data |  8usqMC  8uspC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_42511.map.gz / Format: CCP4 / Size: 75.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_42511.map.gz / Format: CCP4 / Size: 75.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Primary Map (z-flipped) | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.5998 Å | ||||||||||||||||||||||||||||||||||||
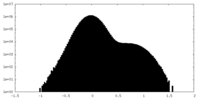
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half map 1
| File | emd_42511_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
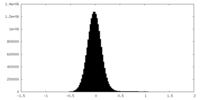
| Density Histograms |
-Half map: #1
| File | emd_42511_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
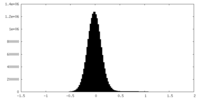
| Density Histograms |
- Sample components
Sample components
-Entire : QUATERNARY COMPLEX OF MET18 TETRAMER
| Entire | Name: QUATERNARY COMPLEX OF MET18 TETRAMER |
|---|---|
| Components |
|
-Supramolecule #1: QUATERNARY COMPLEX OF MET18 TETRAMER
| Supramolecule | Name: QUATERNARY COMPLEX OF MET18 TETRAMER / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: MET18 TETRAMER IMAGED AND SOLVED BY CRYO-EM. |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 118 kDa/nm |
-Macromolecule #1: DNA repair/transcription protein MET18/MMS19
| Macromolecule | Name: DNA repair/transcription protein MET18/MMS19 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 118.007078 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTPDELNSAV VTFMANLNID DSKANETAST VTDSIVHRSI KLLEVVVALK DYFLSENEVE RKKALTCLTT ILAKTPKDHL SKNECSVIF QFYQSKLDDQ ALAKEVLEGF AALAPMKYVS INEIAQLLRL LLDNYQQGQH LASTRLWPFK ILRKIFDRFF V NGSSTEQV ...String: MTPDELNSAV VTFMANLNID DSKANETAST VTDSIVHRSI KLLEVVVALK DYFLSENEVE RKKALTCLTT ILAKTPKDHL SKNECSVIF QFYQSKLDDQ ALAKEVLEGF AALAPMKYVS INEIAQLLRL LLDNYQQGQH LASTRLWPFK ILRKIFDRFF V NGSSTEQV KRINDLFIET FLHVANGEKD PRNLLLSFAL NKSITSSLQN VENFKEDLFD VLFCYFPITF KPPKHDPYKI SN QDLKTAL RSAITATPLF AEDAYSNLLD KLTASSPVVK NDTLLTLLEC VRKFGGSSIL ENWTLLWNAL KFEIMQNSEG NEN TLLNPY NKDQQSDDVG QYTNYDACLK IINLMALQLY NFDKVSFEKF FTHVLDELKP NFKYEKDLKQ TCQILSAIGS GNVE IFNKV ISSTFPLFLI NTSEVAKLKL LIMNFSFFVD SYIDLFGRTS KESLGTPVPN NKMAEYKDEI IMILSMALTR SSKAE VTIR TLSVIQFTKM IKMKGFLTPE EVSLIIQYFT EEILTDNNKN IYYACLEGLK TISEIYEDLV FEISLKKLLD LLPDCF EEK IRVNDEENIH IETILKIILD FTTSRHILVK ESITFLATKL NRVAKISKSR EYCFLLISTI YSLFNNNNQN ENVLNEE DA LALKNAIEPK LFEIITQESA IVSDNYNLTL LSNVLFFTNL KIPQAAHQEE LDRYNELFIS EGKIRILDTP NVLAISYA K ILSALNKNCQ FPQKFTVLFG TVQLLKKHAP RMTETEKLGY LELLLVLSNK FVSEKDVIGL FDWKDLSVIN LEVMVWLTK GLIMQNSLES SEIAKKFIDL LSNEEIGSLV SKLFEVFVMD ISSLKKFKGI SWNNNVKILY KQKFFGDIFQ TLVSNYKNTV DMTIKCNYL TALSLVLKHT PSQSVGPFIN DLFPLLLQAL DMPDPEVRVS ALETLKDTTD KHHTLITEHV STIVPLLLSL S LPHKYNSV SVRLIALQLL EMITTVVPLN YCLSYQDDVL SALIPVLSDK KRIIRKQCVD TRQVYYELGQ IPFE UniProtKB: DNA repair/transcription protein MET18/MMS19 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2 mg/mL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||
| Grid | Material: COPPER / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Atmosphere: OTHER / Pretreatment - Pressure: 0.039 kPa | ||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 77 % / Chamber temperature: 297.15 K / Details: SAMPLE WAS PREPARED ON THE CHAMELEON. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI ARCTICA |
|---|---|
| Software | Name: EPU |
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Average electron dose: 53.47 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 92000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.4 µm / Nominal defocus min: 1.3 µm |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Software | Name: PHENIX |
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
| Output model |  PDB-8usq: |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)