[English] 日本語
 Yorodumi
Yorodumi- EMDB-41317: CryoET reconstruction of 48-nm repeat doublet microtubule from hu... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoET reconstruction of 48-nm repeat doublet microtubule from human sperm | ||||||||||||
 Map data Map data | Composite map for the human dmt48nm combining register 1 and 2. (correct handiness) | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Mammalian sperm / axoneme / microtubule-based structure / microtubule inner protein / non-motor proteins / cellular motility / fertility / structural protein | ||||||||||||
| Biological species |  | ||||||||||||
| Method | subtomogram averaging / cryo EM / Resolution: 10.4 Å | ||||||||||||
 Authors Authors | Chen Z / Shiozak M / Hass KM / Skinner W / Zhao S / Guo C / Polacco BJ / Yu Z / Krogan NJ / Kaake RM ...Chen Z / Shiozak M / Hass KM / Skinner W / Zhao S / Guo C / Polacco BJ / Yu Z / Krogan NJ / Kaake RM / Vale RD / Agard DA | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Cell / Year: 2023 Journal: Cell / Year: 2023Title: De novo protein identification in mammalian sperm using in situ cryoelectron tomography and AlphaFold2 docking. Authors: Zhen Chen / Momoko Shiozaki / Kelsey M Haas / Will M Skinner / Shumei Zhao / Caiying Guo / Benjamin J Polacco / Zhiheng Yu / Nevan J Krogan / Polina V Lishko / Robyn M Kaake / Ronald D Vale / David A Agard /  Abstract: To understand the molecular mechanisms of cellular pathways, contemporary workflows typically require multiple techniques to identify proteins, track their localization, and determine their ...To understand the molecular mechanisms of cellular pathways, contemporary workflows typically require multiple techniques to identify proteins, track their localization, and determine their structures in vitro. Here, we combined cellular cryoelectron tomography (cryo-ET) and AlphaFold2 modeling to address these questions and understand how mammalian sperm are built in situ. Our cellular cryo-ET and subtomogram averaging provided 6.0-Å reconstructions of axonemal microtubule structures. The well-resolved tertiary structures allowed us to unbiasedly match sperm-specific densities with 21,615 AlphaFold2-predicted protein models of the mouse proteome. We identified Tektin 5, CCDC105, and SPACA9 as novel microtubule-associated proteins. These proteins form an extensive interaction network crosslinking the lumen of axonemal doublet microtubules, suggesting their roles in modulating the mechanical properties of the filaments. Indeed, Tekt5 -/- sperm possess more deformed flagella with 180° bends. Together, our studies presented a cellular visual proteomics workflow and shed light on the in vivo functions of Tektin 5. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41317.map.gz emd_41317.map.gz | 18.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41317-v30.xml emd-41317-v30.xml emd-41317.xml emd-41317.xml | 24.8 KB 24.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_41317.png emd_41317.png | 147 KB | ||
| Masks |  emd_41317_msk_1.map emd_41317_msk_1.map | 40.6 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-41317.cif.gz emd-41317.cif.gz | 4.5 KB | ||
| Others |  emd_41317_additional_1.map.gz emd_41317_additional_1.map.gz emd_41317_additional_2.map.gz emd_41317_additional_2.map.gz emd_41317_additional_3.map.gz emd_41317_additional_3.map.gz emd_41317_half_map_1.map.gz emd_41317_half_map_1.map.gz emd_41317_half_map_2.map.gz emd_41317_half_map_2.map.gz | 307 KB 40.8 MB 40.8 MB 31.3 MB 31.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41317 http://ftp.pdbj.org/pub/emdb/structures/EMD-41317 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41317 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41317 | HTTPS FTP |
-Validation report
| Summary document |  emd_41317_validation.pdf.gz emd_41317_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_41317_full_validation.pdf.gz emd_41317_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_41317_validation.xml.gz emd_41317_validation.xml.gz | 12 KB | Display | |
| Data in CIF |  emd_41317_validation.cif.gz emd_41317_validation.cif.gz | 13.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41317 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41317 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41317 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41317 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_41317.map.gz / Format: CCP4 / Size: 80.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41317.map.gz / Format: CCP4 / Size: 80.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Composite map for the human dmt48nm combining register 1 and 2. (correct handiness) | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.65 Å | ||||||||||||||||||||||||||||||||||||
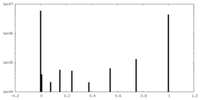
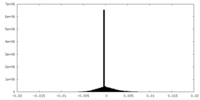
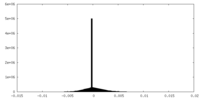
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_41317_msk_1.map emd_41317_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
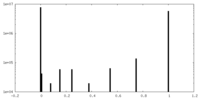
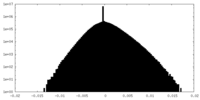
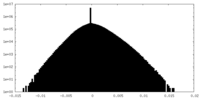
| Density Histograms |
-Additional map: Mask for the register 2 of dmt48 (correct handiness)
| File | emd_41317_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Mask for the register 2 of dmt48 (correct handiness) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Half map1 for the register 2 of dmt48 (correct handiness)
| File | emd_41317_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map1 for the register 2 of dmt48 (correct handiness) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Half map2 for the register 2 of dmt48 (correct handiness)
| File | emd_41317_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map2 for the register 2 of dmt48 (correct handiness) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map1 for the register 1 of dmt48 (correct handiness)
| File | emd_41317_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map1 for the register 1 of dmt48 (correct handiness) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map2 for the register 1 of dmt48 (correct handiness)
| File | emd_41317_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map2 for the register 1 of dmt48 (correct handiness) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Microtubule doublets from mouse sperm flagella treated by EHNA
| Entire | Name: Microtubule doublets from mouse sperm flagella treated by EHNA |
|---|---|
| Components |
|
-Supramolecule #1: Microtubule doublets from mouse sperm flagella treated by EHNA
| Supramolecule | Name: Microtubule doublets from mouse sperm flagella treated by EHNA type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#35 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 30 MDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 4.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 6.0 µm / Nominal defocus min: 2.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 10.4 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION (ver. 4.0-beta2) / Number subtomograms used: 11358 |
|---|---|
| Extraction | Number tomograms: 50 / Number images used: 11358 |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)