+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Apo Bcs1, unsymmetrized | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Heptamer / AAA-ATPase / Translocase | |||||||||
| Function / homology |  Function and homology information Function and homology informationmitochondrial protein-transporting ATPase activity / protein insertion into mitochondrial inner membrane from matrix / mitochondrial cytochrome c oxidase assembly / mitochondrial respiratory chain complex III assembly / mitochondrial respiratory chain complex I assembly / mitochondrial inner membrane / ATP hydrolysis activity / mitochondrion / ATP binding Similarity search - Function | |||||||||
| Biological species |  | |||||||||
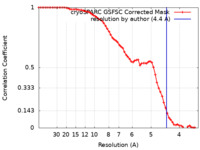
| Method | single particle reconstruction / cryo EM / Resolution: 4.4 Å | |||||||||
 Authors Authors | Zhan J / Xia D | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Conformations of Bcs1L undergoing ATP hydrolysis suggest a concerted translocation mechanism for folded iron-sulfur protein substrate. Authors: Jingyu Zhan / Allison Zeher / Rick Huang / Wai Kwan Tang / Lisa M Jenkins / Di Xia /  Abstract: The human AAA-ATPase Bcs1L translocates the fully assembled Rieske iron-sulfur protein (ISP) precursor across the mitochondrial inner membrane, enabling respiratory Complex III assembly. Exactly how ...The human AAA-ATPase Bcs1L translocates the fully assembled Rieske iron-sulfur protein (ISP) precursor across the mitochondrial inner membrane, enabling respiratory Complex III assembly. Exactly how the folded substrate is bound to and released from Bcs1L has been unclear, and there has been ongoing debate as to whether subunits of Bcs1L act in sequence or in unison hydrolyzing ATP when moving the protein cargo. Here, we captured Bcs1L conformations by cryo-EM during active ATP hydrolysis in the presence or absence of ISP substrate. In contrast to the threading mechanism widely employed by AAA proteins in substrate translocation, subunits of Bcs1L alternate uniformly between ATP and ADP conformations without detectable intermediates that have different, co-existing nucleotide states, indicating that the subunits act in concert. We further show that the ISP can be trapped by Bcs1 when its subunits are all in the ADP-bound state, which we propose to be released in the apo form. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41148.map.gz emd_41148.map.gz | 13.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41148-v30.xml emd-41148-v30.xml emd-41148.xml emd-41148.xml | 15.5 KB 15.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_41148_fsc.xml emd_41148_fsc.xml | 6.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_41148.png emd_41148.png | 64.5 KB | ||
| Filedesc metadata |  emd-41148.cif.gz emd-41148.cif.gz | 5.3 KB | ||
| Others |  emd_41148_additional_1.map.gz emd_41148_additional_1.map.gz emd_41148_half_map_1.map.gz emd_41148_half_map_1.map.gz emd_41148_half_map_2.map.gz emd_41148_half_map_2.map.gz | 25.5 MB 25 MB 25 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41148 http://ftp.pdbj.org/pub/emdb/structures/EMD-41148 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41148 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41148 | HTTPS FTP |
-Validation report
| Summary document |  emd_41148_validation.pdf.gz emd_41148_validation.pdf.gz | 747.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_41148_full_validation.pdf.gz emd_41148_full_validation.pdf.gz | 747.3 KB | Display | |
| Data in XML |  emd_41148_validation.xml.gz emd_41148_validation.xml.gz | 13.7 KB | Display | |
| Data in CIF |  emd_41148_validation.cif.gz emd_41148_validation.cif.gz | 17.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41148 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41148 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41148 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41148 | HTTPS FTP |
-Related structure data
| Related structure data |  8tbyMC  8t14C  8t5uC  8t7uC  8ti0C  8tp1C  8tplC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_41148.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41148.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.72 Å | ||||||||||||||||||||||||||||||||||||
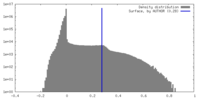
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: #1
| File | emd_41148_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_41148_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_41148_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Heptameric Bcs1 in apo state
| Entire | Name: Heptameric Bcs1 in apo state |
|---|---|
| Components |
|
-Supramolecule #1: Heptameric Bcs1 in apo state
| Supramolecule | Name: Heptameric Bcs1 in apo state / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 340 KDa |
-Macromolecule #1: Mitochondrial chaperone BCS1
| Macromolecule | Name: Mitochondrial chaperone BCS1 / type: protein_or_peptide / ID: 1 / Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 48.289887 KDa |
| Recombinant expression | Organism:  Komagataella pastoris (fungus) Komagataella pastoris (fungus) |
| Sequence | String: MPFSDFVLAL KDNPYFGAGF GLVGVGTALA MARKGAQLGL VAFRRHYMIT LEVPARDRSY AWLLSWLTRH STRTQHLSVE TSYLQHESG RISTKFEFIP SPGNHFIWYQ GKWIRVERNR DMQMVDLQTG TPWESVTFTA LGTDRKVFFN ILEEARALAL Q QEEGKTVM ...String: MPFSDFVLAL KDNPYFGAGF GLVGVGTALA MARKGAQLGL VAFRRHYMIT LEVPARDRSY AWLLSWLTRH STRTQHLSVE TSYLQHESG RISTKFEFIP SPGNHFIWYQ GKWIRVERNR DMQMVDLQTG TPWESVTFTA LGTDRKVFFN ILEEARALAL Q QEEGKTVM YTAVGSEWRT FGYPRRRRPL DSVVLQQGLA DRIVKDIREF IDNPKWYIDR GIPYRRGYLL YGPPGCGKSS FI TALAGEL EHSICLLSLT DSSLSDDRLN HLLSVAPQQS LVLLEDVDAA FLSRDLAVEN PIKYQGLGRL TFSGLLNALD GVA STEARI VFMTTNYIDR LDPALIRPGR VDLKEYVGYC SHWQLTQMFQ RFYPGQAPSL AENFAEHVLK ATSEISPAQV QGYF MLYKN DPMGAVHNIE SLRHHHHHH UniProtKB: Mitochondrial chaperone BCS1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.25 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)