+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the amthamine-bound H2R-Gs complex | |||||||||
 Map data Map data | Sharpened Map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GPCR / H2R / Histamine Receptor / MEMBRANE PROTEIN/IMMUNE SYSTEM / MEMBRANE PROTEIN-IMMUNE SYSTEM complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationgastric acid secretion / Histamine receptors / histamine receptor activity / G protein-coupled serotonin receptor activity / neurotransmitter receptor activity / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / positive regulation of vasoconstriction / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta ...gastric acid secretion / Histamine receptors / histamine receptor activity / G protein-coupled serotonin receptor activity / neurotransmitter receptor activity / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / positive regulation of vasoconstriction / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G-protein activation / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through CDC42 / ADP signalling through P2Y purinoceptor 12 / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / Sensory perception of sweet, bitter, and umami (glutamate) taste / photoreceptor disc membrane / Adrenaline,noradrenaline inhibits insulin secretion / Glucagon-type ligand receptors / Vasopressin regulates renal water homeostasis via Aquaporins / G alpha (z) signalling events / cellular response to catecholamine stimulus / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / ADORA2B mediated anti-inflammatory cytokines production / sensory perception of taste / ADP signalling through P2Y purinoceptor 1 / adenylate cyclase-activating dopamine receptor signaling pathway / G beta:gamma signalling through PI3Kgamma / cellular response to prostaglandin E stimulus / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / G-protein beta-subunit binding / Inactivation, recovery and regulation of the phototransduction cascade / heterotrimeric G-protein complex / G alpha (12/13) signalling events / extracellular vesicle / signaling receptor complex adaptor activity / Thrombin signalling through proteinase activated receptors (PARs) / GTPase binding / retina development in camera-type eye / phospholipase C-activating G protein-coupled receptor signaling pathway / Ca2+ pathway / G alpha (i) signalling events / fibroblast proliferation / G alpha (s) signalling events / G alpha (q) signalling events / chemical synaptic transmission / cell population proliferation / Ras protein signal transduction / Extra-nuclear estrogen signaling / immune response / G protein-coupled receptor signaling pathway / lysosomal membrane / GTPase activity / synapse / dendrite / protein-containing complex binding / signal transduction / extracellular exosome / membrane / plasma membrane / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / synthetic construct (others) Homo sapiens (human) / synthetic construct (others) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.7 Å | |||||||||
 Authors Authors | Shen Q / Tang X / Wen X / Cheng S / Xiao P / Zang S / Shen D / Jiang L / Zheng Y / Zhang H ...Shen Q / Tang X / Wen X / Cheng S / Xiao P / Zang S / Shen D / Jiang L / Zheng Y / Zhang H / Xu H / Mao C / Zhang M / Hu W / Sun J / Chen Z / Zhang Y | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Adv Sci (Weinh) / Year: 2024 Journal: Adv Sci (Weinh) / Year: 2024Title: Molecular Determinant Underlying Selective Coupling of Primary G-Protein by Class A GPCRs. Authors: Qingya Shen / Xinyan Tang / Xin Wen / Shizhuo Cheng / Peng Xiao / Shao-Kun Zang / Dan-Dan Shen / Lei Jiang / Yanrong Zheng / Huibing Zhang / Haomang Xu / Chunyou Mao / Min Zhang / Weiwei Hu ...Authors: Qingya Shen / Xinyan Tang / Xin Wen / Shizhuo Cheng / Peng Xiao / Shao-Kun Zang / Dan-Dan Shen / Lei Jiang / Yanrong Zheng / Huibing Zhang / Haomang Xu / Chunyou Mao / Min Zhang / Weiwei Hu / Jin-Peng Sun / Yan Zhang / Zhong Chen /  Abstract: G-protein-coupled receptors (GPCRs) transmit downstream signals predominantly via G-protein pathways. However, the conformational basis of selective coupling of primary G-protein remains elusive. ...G-protein-coupled receptors (GPCRs) transmit downstream signals predominantly via G-protein pathways. However, the conformational basis of selective coupling of primary G-protein remains elusive. Histamine receptors HR and HR couple with G- or G-proteins respectively. Here, three cryo-EM structures of HR-G and HR-G complexes are presented at a global resolution of 2.6-2.7 Å. These structures reveal the unique binding pose for endogenous histamine in HR, wherein the amino group interacts with E206 of HR instead of the conserved D114 of other aminergic receptors. Furthermore, comparative analysis of the HR-G and HR-G complexes reveals that the structural geometry of TM5/TM6 determines the primary G-protein selectivity in histamine receptors. Machine learning (ML)-based structuromic profiling and functional analysis of class A GPCR-G-protein complexes illustrate that TM5 length, TM5 tilt, and TM6 outward movement are key determinants of the G and G selectivity among the whole Class A family. Collectively, the findings uncover the common structural geometry within class A GPCRs that determines the primary G- and G-coupling selectivity. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_39582.map.gz emd_39582.map.gz | 17.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-39582-v30.xml emd-39582-v30.xml emd-39582.xml emd-39582.xml | 20.4 KB 20.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_39582.png emd_39582.png | 148 KB | ||
| Filedesc metadata |  emd-39582.cif.gz emd-39582.cif.gz | 6.6 KB | ||
| Others |  emd_39582_half_map_1.map.gz emd_39582_half_map_1.map.gz emd_39582_half_map_2.map.gz emd_39582_half_map_2.map.gz | 20.6 MB 20.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-39582 http://ftp.pdbj.org/pub/emdb/structures/EMD-39582 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39582 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39582 | HTTPS FTP |
-Validation report
| Summary document |  emd_39582_validation.pdf.gz emd_39582_validation.pdf.gz | 720.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_39582_full_validation.pdf.gz emd_39582_full_validation.pdf.gz | 720 KB | Display | |
| Data in XML |  emd_39582_validation.xml.gz emd_39582_validation.xml.gz | 10.4 KB | Display | |
| Data in CIF |  emd_39582_validation.cif.gz emd_39582_validation.cif.gz | 12.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39582 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39582 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39582 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39582 | HTTPS FTP |
-Related structure data
| Related structure data |  8yutMC  8yuuC  8yuvC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_39582.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_39582.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened Map | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.014 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: half map 1
| File | emd_39582_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 1 | ||||||||||||
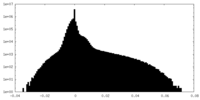
| Projections & Slices |
| ||||||||||||
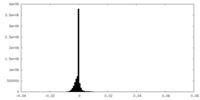
| Density Histograms |
-Half map: half map2
| File | emd_39582_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map2 | ||||||||||||
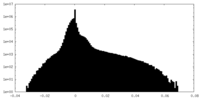
| Projections & Slices |
| ||||||||||||
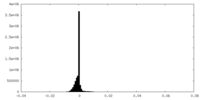
| Density Histograms |
- Sample components
Sample components
-Entire : amthamine-bound H2R-Gs complex
| Entire | Name: amthamine-bound H2R-Gs complex |
|---|---|
| Components |
|
-Supramolecule #1: amthamine-bound H2R-Gs complex
| Supramolecule | Name: amthamine-bound H2R-Gs complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short
| Macromolecule | Name: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 43.883762 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGCLGNSKTE DQRNEEKAQR EANKKIEKQL QKDKQVYRAT HRLLLLGAGE SGKSTIVKQM RILHVNGYSE EECKQYKAVV YSNTIQSII AIIRAMGRLK IDFGDSARAD DARQLFVLAG AAEEGFMTAE LAGVIKRLWK DSGVQACFNR SREYQLNDSA A YYLNDLDR ...String: MGCLGNSKTE DQRNEEKAQR EANKKIEKQL QKDKQVYRAT HRLLLLGAGE SGKSTIVKQM RILHVNGYSE EECKQYKAVV YSNTIQSII AIIRAMGRLK IDFGDSARAD DARQLFVLAG AAEEGFMTAE LAGVIKRLWK DSGVQACFNR SREYQLNDSA A YYLNDLDR IAQPNYIPTQ QDVLRTRVKT SGIFETKFQV DKVNFHMFDV GAQRDERRKW IQCFNDVTAI IFVVASSSYN MV IREDNQT NRLQEALNLF KSIWNNRWLR TISVILFLNK QDLLAEKVLA GKSKIEDYFP EFARYTTPED ATPEPGEDPR VTR AKYFIR DEFLRISTAS GDGRHYCYPH FTCSVDTENI RRVFNDCRDI IQRMHLRQYE LL |
-Macromolecule #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 39.141793 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MHHHHLEVLF QGPGSSGSEL DQLRQEAEQL KNQIRDARKA CADATLSQIT NNIDPVGRIQ MRTRRTLRGH LAKIYAMHWG TDSRLLVSA SQDGKLIIWD SYTTNKVHAI PLRSSWVMTC AYAPSGNYVA CGGLDNICSI YNLKTREGNV RVSRELAGHT G YLSCCRFL ...String: MHHHHLEVLF QGPGSSGSEL DQLRQEAEQL KNQIRDARKA CADATLSQIT NNIDPVGRIQ MRTRRTLRGH LAKIYAMHWG TDSRLLVSA SQDGKLIIWD SYTTNKVHAI PLRSSWVMTC AYAPSGNYVA CGGLDNICSI YNLKTREGNV RVSRELAGHT G YLSCCRFL DDNQIVTSSG DTTCALWDIE TGQQTTTFTG HTGDVMSLSL APDTRLFVSG ACDASAKLWD VREGMCRQTF TG HESDINA ICFFPNGNAF ATGSDDATCR LFDLRADQEL MTYSHDNIIC GITSVSFSKS GRLLLAGYDD FNCNVWDALK ADR AGVLAG HDNRVSCLGV TDDGMAVATG SWDSFLKIWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #4: Nanobody-35
| Macromolecule | Name: Nanobody-35 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 13.885439 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QVQLQESGGG LVQPGGSLRL SCAASGFTFS NYKMNWVRQA PGKGLEWVSD ISQSGASISY TGSVKGRFTI SRDNAKNTLY LQMNSLKPE DTAVYYCARC PAPFTRDCFD VTSTTYAYRG QGTQVTVSS |
-Macromolecule #5: Histamine H2 receptor
| Macromolecule | Name: Histamine H2 receptor / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 46.456023 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKTIIALSYI FCLVFADYKD DDDKEFLEVL FQGPAPNGTA SSFCLDSTAC KITITVVLAV LILITVAGNV VVCLAVGLNR RLRNLTNCF IVSLAITDLL LGLLVLPFSA IYQLSCKWSF GKVFCNIYTS LDVMLCTASI LNLFMISLDR YCAVMDPLRY P VLVTPVRV ...String: MKTIIALSYI FCLVFADYKD DDDKEFLEVL FQGPAPNGTA SSFCLDSTAC KITITVVLAV LILITVAGNV VVCLAVGLNR RLRNLTNCF IVSLAITDLL LGLLVLPFSA IYQLSCKWSF GKVFCNIYTS LDVMLCTASI LNLFMISLDR YCAVMDPLRY P VLVTPVRV AISLVLIWVI SITLSFLSIH LGWNSRNETS KGNHTTSKCK VQVNEVYGLV DGLVTFYLPL LIMCITYYRI FK VARDQAK RINHISSWKA ATIREHKATV TLAAVMGAFI ICWFPYFTAF VYRGLRGDDA INEVLEAIVL WLGYANSALN PIL YAALNR DFRTGYQQLF CCRLANRNSH KTSLRSNASQ LSRTQSREPR QQEEKPLKLQ VWSGTEVTAP QGATDRLEEN LYFQ GHHHH HHHHHH UniProtKB: Histamine H2 receptor |
-Macromolecule #6: 5-(2-azanylethyl)-4-methyl-1,3-thiazol-2-amine
| Macromolecule | Name: 5-(2-azanylethyl)-4-methyl-1,3-thiazol-2-amine / type: ligand / ID: 6 / Number of copies: 1 / Formula: A1D67 |
|---|---|
| Molecular weight | Theoretical: 157.237 Da |
-Macromolecule #7: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 7 / Number of copies: 1 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 62.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.7 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 368447 |
| Initial angle assignment | Type: RANDOM ASSIGNMENT |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
|---|---|
| Output model |  PDB-8yut: |
 Movie
Movie Controller
Controller



























 Z
Z Y
Y X
X