+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human urea transporter A2. | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Urea transporter / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationurea transport / Transport of bile salts and organic acids, metal ions and amine compounds / urea transmembrane transport / urea transmembrane transporter activity / cell adhesion molecule binding / transmembrane transport / apical plasma membrane / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  Homo sapien (human) Homo sapien (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Huang S / Liu L / Sun J | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structural insights into the mechanisms of urea permeation and distinct inhibition modes of urea transporters. Authors: Shen-Ming Huang / Zhi-Zhen Huang / Lei Liu / Meng-Yao Xiong / Chao Zhang / Bo-Yang Cai / Ming-Wei Wang / Kui Cai / Ying-Li Jia / Jia-Le Wang / Ming-Hui Zhang / Yi-He Xie / Min Li / Hang ...Authors: Shen-Ming Huang / Zhi-Zhen Huang / Lei Liu / Meng-Yao Xiong / Chao Zhang / Bo-Yang Cai / Ming-Wei Wang / Kui Cai / Ying-Li Jia / Jia-Le Wang / Ming-Hui Zhang / Yi-He Xie / Min Li / Hang Zhang / Cheng-Hao Weng / Xin Wen / Zhi Li / Ying Sun / Fan Yi / Zhao Yang / Peng Xiao / Fan Yang / Xiao Yu / Lu Tie / Bao-Xue Yang / Jin-Peng Sun /  Abstract: Urea's transmembrane transport through urea transporters (UT) is a fundamental physiological behavior for life activities. Here, we present 11 cryo-EM structures of four UT members in resting states, ...Urea's transmembrane transport through urea transporters (UT) is a fundamental physiological behavior for life activities. Here, we present 11 cryo-EM structures of four UT members in resting states, urea transport states, or inactive states bound with synthetic competitive, uncompetitive or noncompetitive inhibitor. Our results indicate that the binding of urea via a conserved urea recognition motif (URM) and the urea transport via H-bond transfer along the Q-T-T-Q motif among different UT members. Moreover, distinct binding modes of the competitive inhibitors 25a and ATB3, the uncompetitive inhibitor CF11 and the noncompetitive inhibitor HQA2 provide different mechanisms for blocking urea transport and achieved selectivity through L-P pocket, UCBP region and SCG pocket, respectively. In summary, our study not only allows structural understanding of urea transport via UTs but also afforded a structural landscape of hUT-A2 inhibition by competitive, uncompetitive and noncompetitive inhibitors, which may facilitate developing selective human UT-A inhibitors as a new class of salt-sparing diuretics. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_38274.map.gz emd_38274.map.gz | 46.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-38274-v30.xml emd-38274-v30.xml emd-38274.xml emd-38274.xml | 15.1 KB 15.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_38274.png emd_38274.png | 227.4 KB | ||
| Filedesc metadata |  emd-38274.cif.gz emd-38274.cif.gz | 5.6 KB | ||
| Others |  emd_38274_half_map_1.map.gz emd_38274_half_map_1.map.gz emd_38274_half_map_2.map.gz emd_38274_half_map_2.map.gz | 84.6 MB 84.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-38274 http://ftp.pdbj.org/pub/emdb/structures/EMD-38274 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38274 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38274 | HTTPS FTP |
-Validation report
| Summary document |  emd_38274_validation.pdf.gz emd_38274_validation.pdf.gz | 915.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_38274_full_validation.pdf.gz emd_38274_full_validation.pdf.gz | 915.1 KB | Display | |
| Data in XML |  emd_38274_validation.xml.gz emd_38274_validation.xml.gz | 13.3 KB | Display | |
| Data in CIF |  emd_38274_validation.cif.gz emd_38274_validation.cif.gz | 15.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-38274 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-38274 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-38274 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-38274 | HTTPS FTP |
-Related structure data
| Related structure data |  8xddMC  8xd7C  8xd9C  8xdaC  8xdbC  8xdcC  8xdeC  8xdfC  8xdgC  8xdhC  8xdiC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_38274.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_38274.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.82 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_38274_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_38274_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Homotrimer complex of human urea transporter
| Entire | Name: Homotrimer complex of human urea transporter |
|---|---|
| Components |
|
-Supramolecule #1: Homotrimer complex of human urea transporter
| Supramolecule | Name: Homotrimer complex of human urea transporter / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Urea transporter 2
| Macromolecule | Name: Urea transporter 2 / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapien (human) Homo sapien (human) |
| Molecular weight | Theoretical: 43.419789 KDa |
| Recombinant expression | Organism:  Spodoptera (butterflies/moths) Spodoptera (butterflies/moths) |
| Sequence | String: MEESSEIKVE TNISKTSWIR SSMAASGKRV SKALSYITGE MKECGEGLKD KSPVFQFFDW VLRGTSQVMF VNNPLSGILI ILGLFIQNP WWAISGCLGT IMSTLTALIL SQDKSAIAAG FHGYNGVLVG LLMAVFSDKG DYYWWLLLPV IIMSMSCPIL S SALGTIFS ...String: MEESSEIKVE TNISKTSWIR SSMAASGKRV SKALSYITGE MKECGEGLKD KSPVFQFFDW VLRGTSQVMF VNNPLSGILI ILGLFIQNP WWAISGCLGT IMSTLTALIL SQDKSAIAAG FHGYNGVLVG LLMAVFSDKG DYYWWLLLPV IIMSMSCPIL S SALGTIFS KWDLPVFTLP FNITVTLYLA ATGHYNLFFP TTLLQPASAM PNITWSEVQV PLLLRAIPVG IGQVYGCDNP WT GGIFLIA LFISSPLICL HAAIGSTMGM LAALTIATPF DSIYFGLCGF NSTLACIAIG GMFYVITWQT HLLAIACALF AAY LGAALA NMLSVFGLPP CTWPFCLSAL TFLLLTTNNP AIYKLPLSKV TYPEANRIYY LSQERNRRAS IITKYQAYDV S UniProtKB: Urea transporter 2 |
-Macromolecule #2: 8-hydroxyquinoline-2-carboxylic acid
| Macromolecule | Name: 8-hydroxyquinoline-2-carboxylic acid / type: ligand / ID: 2 / Number of copies: 3 / Formula: 8HC |
|---|---|
| Molecular weight | Theoretical: 189.167 Da |
| Chemical component information | 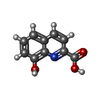 ChemComp-8HC: |
-Macromolecule #3: 1-(3-methoxyphenyl)methanamine
| Macromolecule | Name: 1-(3-methoxyphenyl)methanamine / type: ligand / ID: 3 / Number of copies: 3 / Formula: SZ4 |
|---|---|
| Molecular weight | Theoretical: 137.179 Da |
| Chemical component information | 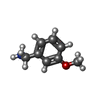 ChemComp-SZ4: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: FREON 12 |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source: OTHER |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: OTHER / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.0 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 92767 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: OTHER |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)