+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | ADP-bound purinergic receptor 1 in complex with miniGs/q | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GPCR / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationG protein-coupled ATP receptor activity / relaxation of muscle / G protein-coupled ADP receptor activity / P2Y receptors / G protein-coupled purinergic nucleotide receptor activity / positive regulation of inositol trisphosphate biosynthetic process / cellular response to purine-containing compound / A1 adenosine receptor binding / negative regulation of norepinephrine secretion / positive regulation of monoatomic ion transport ...G protein-coupled ATP receptor activity / relaxation of muscle / G protein-coupled ADP receptor activity / P2Y receptors / G protein-coupled purinergic nucleotide receptor activity / positive regulation of inositol trisphosphate biosynthetic process / cellular response to purine-containing compound / A1 adenosine receptor binding / negative regulation of norepinephrine secretion / positive regulation of monoatomic ion transport / positive regulation of penile erection / glial cell migration / regulation of presynaptic cytosolic calcium ion concentration / G protein-coupled adenosine receptor signaling pathway / signal transduction involved in regulation of gene expression / positive regulation of hormone secretion / response to growth factor / regulation of synaptic vesicle exocytosis / cellular response to ATP / eating behavior / response to mechanical stimulus / monoatomic ion transport / presynaptic active zone membrane / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / blood vessel diameter maintenance / establishment of localization in cell / protein localization to plasma membrane / electron transport chain / ADP binding / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / cilium / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / platelet activation / G-protein activation / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through CDC42 / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / photoreceptor disc membrane / Glucagon-type ligand receptors / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / G alpha (z) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / sensory perception of taste / ADP signalling through P2Y purinoceptor 1 / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / cellular response to prostaglandin E stimulus / Inactivation, recovery and regulation of the phototransduction cascade / G-protein beta-subunit binding / heterotrimeric G-protein complex / G alpha (12/13) signalling events / extracellular vesicle / signaling receptor complex adaptor activity / Thrombin signalling through proteinase activated receptors (PARs) / GTPase binding / retina development in camera-type eye / signaling receptor activity / regulation of cell shape / Ca2+ pathway / phospholipase C-activating G protein-coupled receptor signaling pathway / positive regulation of cytosolic calcium ion concentration / cell body / G alpha (i) signalling events / scaffold protein binding / fibroblast proliferation / G alpha (s) signalling events / basolateral plasma membrane / G alpha (q) signalling events / postsynaptic membrane / Ras protein signal transduction / cell population proliferation / Extra-nuclear estrogen signaling / periplasmic space / positive regulation of ERK1 and ERK2 cascade / electron transfer activity / postsynaptic density / cell surface receptor signaling pathway / iron ion binding / protein heterodimerization activity / positive regulation of protein phosphorylation / G protein-coupled receptor signaling pathway / apical plasma membrane / lysosomal membrane / GTPase activity / glutamatergic synapse Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Gu QC / Tang WQ | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: ADP-bound purinergic receptor 1 in complex with miniGs/q Authors: Gu QC / Tang WQ #1:  Journal: To Be Published Journal: To Be PublishedTitle: Real-space refinement in PHENIX for cryo-EM and crystallography Authors: Gu QC / Tang WQ | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_37591.map.gz emd_37591.map.gz | 59.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-37591-v30.xml emd-37591-v30.xml emd-37591.xml emd-37591.xml | 19 KB 19 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_37591_fsc.xml emd_37591_fsc.xml | 7.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_37591.png emd_37591.png | 31.4 KB | ||
| Masks |  emd_37591_msk_1.map emd_37591_msk_1.map | 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-37591.cif.gz emd-37591.cif.gz | 6.5 KB | ||
| Others |  emd_37591_half_map_1.map.gz emd_37591_half_map_1.map.gz emd_37591_half_map_2.map.gz emd_37591_half_map_2.map.gz | 59.5 MB 59.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-37591 http://ftp.pdbj.org/pub/emdb/structures/EMD-37591 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37591 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37591 | HTTPS FTP |
-Validation report
| Summary document |  emd_37591_validation.pdf.gz emd_37591_validation.pdf.gz | 929.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_37591_full_validation.pdf.gz emd_37591_full_validation.pdf.gz | 928.8 KB | Display | |
| Data in XML |  emd_37591_validation.xml.gz emd_37591_validation.xml.gz | 15.2 KB | Display | |
| Data in CIF |  emd_37591_validation.cif.gz emd_37591_validation.cif.gz | 20.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37591 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37591 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37591 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37591 | HTTPS FTP |
-Related structure data
| Related structure data |  8wjxMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_37591.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_37591.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1 Å | ||||||||||||||||||||||||||||||||||||
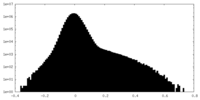
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_37591_msk_1.map emd_37591_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
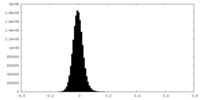
| Density Histograms |
-Half map: #2
| File | emd_37591_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_37591_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : ADP-bound purinergic receptor 1 in complex with miniGs/q
| Entire | Name: ADP-bound purinergic receptor 1 in complex with miniGs/q |
|---|---|
| Components |
|
-Supramolecule #1: ADP-bound purinergic receptor 1 in complex with miniGs/q
| Supramolecule | Name: ADP-bound purinergic receptor 1 in complex with miniGs/q type: complex / ID: 1 / Parent: 0 / Macromolecule list: #5, #1, #4, #2-#3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: GNAS complex locus
| Macromolecule | Name: GNAS complex locus / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 30.840807 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGMGHHHHHH ENLYFQGNSK TEDQRNEEKA QREANKKIEK QLQKDKQVYR ATHRLLLLGA DNSGKSTIVK QMRILHGGSG GSGGTSGIF ETKFQVDKVN FHMFDVGGQR DERRKWIQCF NDVTAIIFVV DSSDYNRLQE ALNLFKSIWN NRWLRTISVI L FLNKQDLL ...String: MGMGHHHHHH ENLYFQGNSK TEDQRNEEKA QREANKKIEK QLQKDKQVYR ATHRLLLLGA DNSGKSTIVK QMRILHGGSG GSGGTSGIF ETKFQVDKVN FHMFDVGGQR DERRKWIQCF NDVTAIIFVV DSSDYNRLQE ALNLFKSIWN NRWLRTISVI L FLNKQDLL AEKVLAGKSK IEDYFPEFAR YTTPEDATPE PGEDPRVTRA KYFIRDEFLR ISTASGDGRH YCYPHFTCAV DT ENARRIF NDCKDIILQM NLREYNLV |
-Macromolecule #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 41.055867 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MHHHHHHGSL LQSELDQLRQ EAEQLKNQIR DARKACADAT LSQITNNIDP VGRIQMRTRR TLRGHLAKIY AMHWGTDSRL LVSASQDGK LIIWDSYTTN KVHAIPLRSS WVMTCAYAPS GNYVACGGLD NICSIYNLKT REGNVRVSRE LAGHTGYLSC C RFLDDNQI ...String: MHHHHHHGSL LQSELDQLRQ EAEQLKNQIR DARKACADAT LSQITNNIDP VGRIQMRTRR TLRGHLAKIY AMHWGTDSRL LVSASQDGK LIIWDSYTTN KVHAIPLRSS WVMTCAYAPS GNYVACGGLD NICSIYNLKT REGNVRVSRE LAGHTGYLSC C RFLDDNQI VTSSGDTTCA LWDIETGQQT TTFTGHTGDV MSLSLAPDTR LFVSGACDAS AKLWDVREGM CRQTFTGHES DI NAICFFP NGNAFATGSD DATCRLFDLR ADQELMTYSH DNIICGITSV SFSKSGRLLL AGYDDFNCNV WDALKADRAG VLA GHDNRV SCLGVTDDGM AVATGSWDSF LKIWNGSSGG GGSGGGGSSG VSGWRLFKKI S UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #4: Nanobody 35
| Macromolecule | Name: Nanobody 35 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 17.057271 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKYLLPTAAA GLLLLAAQPA MAMQVQLQES GGGLVQPGGS LRLSCAASGF TFSNYKMNWV RQAPGKGLEW VSDISQSGAS ISYTGSVKG RFTISRDNAK NTLYLQMNSL KPEDTAVYYC ARCPAPFTRD CFDVTSTTYA YRGQGTQVTV SSHHHHHH |
-Macromolecule #5: Soluble cytochrome b562,P2Y purinoceptor 1,Soluble cytochrome b56...
| Macromolecule | Name: Soluble cytochrome b562,P2Y purinoceptor 1,Soluble cytochrome b562,P2Y purinoceptor 1 type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 76.586242 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKTIIALSYI FCLVFADYKD DDDAGRAHHH HHHHHHHENL YFQSGAPADL EDNWETLNDN LKVIEKADNA AQVKDALTKM RAAALDAQK ATPPKLEDKS PDSPEMKDFR HGFDILVGQI DDALKLANEG KVKEAQAAAE QLKTTRNAYI QKYLEFLEVL F QGPTEVLW ...String: MKTIIALSYI FCLVFADYKD DDDAGRAHHH HHHHHHHENL YFQSGAPADL EDNWETLNDN LKVIEKADNA AQVKDALTKM RAAALDAQK ATPPKLEDKS PDSPEMKDFR HGFDILVGQI DDALKLANEG KVKEAQAAAE QLKTTRNAYI QKYLEFLEVL F QGPTEVLW PAVPNGTDAA FLAGPGSSWG NSTVASTAAV SSSFKCALTK TGFQFYYLPA VYILVFIIGF LGNSVAIWMF VF HMKPWSG ISVYMFNLAL ADFLYVLTLP ALIFYYFNKT DWIFGDAMCK LQRFIFHVNL YGSILFLTCI SAHRYSGVVY PLK SLGRLK KKNAICISVL VWLIVVVAIS PILFYSGTGV RKNKTITCYD TTSDEYLRSY FIYSMCTTVA MFCVPLVLIL GCYG LIVRA LIYKDLDNSP LRRKSIYLVI IVLTVFAVSY IPFHVMKTMN LRARLDFQTP AMCAFNDRVY ATYQVTRGLA SLNSC VDPI LYFLAGDTFR RRLSRATRKA SRRSEANLQS KSEDMTLNVF TLEDFVGDWE QTAAYNLDQV LEQGGVSSLL QNLAVS VTP IQRIVRSGEN ALKIDIHVII PYEGLSADQM AQIEEVFKVV YPVDDHHFKV ILPYGTLVID GVTPNMLNYF GRPYEGI AV FDGKKITVTG TLWNGNKIID ERLITPDGSM LFRVTINS UniProtKB: Soluble cytochrome b562, P2Y purinoceptor 1 |
-Macromolecule #6: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 6 / Number of copies: 1 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #7: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 7 / Number of copies: 1 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)