[English] 日本語
 Yorodumi
Yorodumi- EMDB-37417: CryoEM structure of Snf7 N-terminal domain in the inner coils of ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of Snf7 N-terminal domain in the inner coils of spiral | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | membrane fission / spiral polymers / N-terminal domain / "open" status / PROTEIN TRANSPORT | |||||||||
| Function / homology | ESCRT III complex / Snf7 family / Snf7 / late endosome to vacuole transport via multivesicular body sorting pathway / vesicle budding from membrane / multivesicular body / cytoplasmic side of plasma membrane / Vacuolar-sorting protein SNF7 Function and homology information Function and homology information | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 7.4 Å | |||||||||
 Authors Authors | Liu MD / Shen QT | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2024 Journal: Proc Natl Acad Sci U S A / Year: 2024Title: Three-dimensional architecture of ESCRT-III flat spirals on the membrane. Authors: Mingdong Liu / Yunhui Liu / Tiefeng Song / Liuyan Yang / Lei Qi / Yu-Zhong Zhang / Yong Wang / Qing-Tao Shen /  Abstract: The endosomal sorting complexes required for transport (ESCRTs) are responsible for membrane remodeling in many cellular processes, such as multivesicular body biogenesis, viral budding, and ...The endosomal sorting complexes required for transport (ESCRTs) are responsible for membrane remodeling in many cellular processes, such as multivesicular body biogenesis, viral budding, and cytokinetic abscission. ESCRT-III, the most abundant ESCRT subunit, assembles into flat spirals as the primed state, essential to initiate membrane invagination. However, the three-dimensional architecture of ESCRT-III flat spirals remained vague for decades due to highly curved filaments with a small diameter and a single preferred orientation on the membrane. Here, we unveiled that yeast Snf7, a component of ESCRT-III, forms flat spirals on the lipid monolayers using cryogenic electron microscopy. We developed a geometry-constrained Euler angle-assigned reconstruction strategy and obtained moderate-resolution structures of Snf7 flat spirals with varying curvatures. Our analyses showed that Snf7 subunits recline on the membrane with N-terminal motifs α0 as anchors, adopt an open state with fused α2/3 helices, and bend α2/3 gradually from the outer to inner parts of flat spirals. In all, we provide the orientation and conformations of ESCRT-III flat spirals on the membrane and unveil the underlying assembly mechanism, which will serve as the initial step in understanding how ESCRTs drive membrane abscission. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_37417.map.gz emd_37417.map.gz | 11.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-37417-v30.xml emd-37417-v30.xml emd-37417.xml emd-37417.xml | 13.2 KB 13.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_37417.png emd_37417.png | 15 KB | ||
| Filedesc metadata |  emd-37417.cif.gz emd-37417.cif.gz | 5.1 KB | ||
| Others |  emd_37417_half_map_1.map.gz emd_37417_half_map_1.map.gz emd_37417_half_map_2.map.gz emd_37417_half_map_2.map.gz | 11.9 MB 11.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-37417 http://ftp.pdbj.org/pub/emdb/structures/EMD-37417 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37417 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37417 | HTTPS FTP |
-Validation report
| Summary document |  emd_37417_validation.pdf.gz emd_37417_validation.pdf.gz | 837.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_37417_full_validation.pdf.gz emd_37417_full_validation.pdf.gz | 837.2 KB | Display | |
| Data in XML |  emd_37417_validation.xml.gz emd_37417_validation.xml.gz | 9.6 KB | Display | |
| Data in CIF |  emd_37417_validation.cif.gz emd_37417_validation.cif.gz | 11.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37417 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37417 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37417 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37417 | HTTPS FTP |
-Related structure data
| Related structure data |  8wb7MC  8wb6C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_37417.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_37417.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||
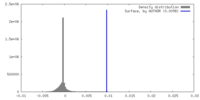
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_37417_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
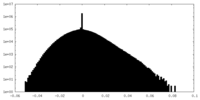
| Projections & Slices |
| ||||||||||||
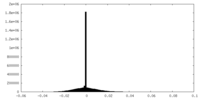
| Density Histograms |
-Half map: #2
| File | emd_37417_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
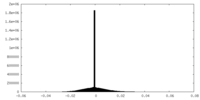
| Density Histograms |
- Sample components
Sample components
-Entire : Snf7 spiral polymers
| Entire | Name: Snf7 spiral polymers |
|---|---|
| Components |
|
-Supramolecule #1: Snf7 spiral polymers
| Supramolecule | Name: Snf7 spiral polymers / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: SNF7 isoform 1
| Macromolecule | Name: SNF7 isoform 1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 27.020119 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MWSSLFGWTS SNAKNKESPT KAIVRLREHI NLLSKKQSHL RTQITNQENE ARIFLTKGNK VMAKNALKKK KTIEQLLSKV EGTMESMEQ QLFSIESANL NLETMRAMQE GAKAMKTIHS GLDIDKVDET MDEIREQVEL GDEISDAISR PLITGANEVD E DELDEELD ...String: MWSSLFGWTS SNAKNKESPT KAIVRLREHI NLLSKKQSHL RTQITNQENE ARIFLTKGNK VMAKNALKKK KTIEQLLSKV EGTMESMEQ QLFSIESANL NLETMRAMQE GAKAMKTIHS GLDIDKVDET MDEIREQVEL GDEISDAISR PLITGANEVD E DELDEELD MLAQENANQE TSKIVNNNVN AAPISENKVS LPSVPSNKIK QSENSVKDGE EEEDEEDEDE KALRELQAEM GL UniProtKB: Vacuolar-sorting protein SNF7 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.6 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.4 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 7.4 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 187135 |
| Initial angle assignment | Type: OTHER |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)