+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of CUL3-RBX1-KLHL22 complex without CUL3 NA motif | |||||||||||||||
 Map data Map data | ||||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | Cullin Ring E3 ubiquitin ligase / LIGASE | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationliver morphogenesis / positive regulation of mitotic cell cycle phase transition / trophectodermal cellular morphogenesis / POZ domain binding / nuclear protein quality control by the ubiquitin-proteasome system / regulation protein catabolic process at postsynapse / polar microtubule / anaphase-promoting complex-dependent catabolic process / COPII vesicle coating / cullin-RING-type E3 NEDD8 transferase ...liver morphogenesis / positive regulation of mitotic cell cycle phase transition / trophectodermal cellular morphogenesis / POZ domain binding / nuclear protein quality control by the ubiquitin-proteasome system / regulation protein catabolic process at postsynapse / polar microtubule / anaphase-promoting complex-dependent catabolic process / COPII vesicle coating / cullin-RING-type E3 NEDD8 transferase / stem cell division / cellular response to chemical stress / NEDD8 transferase activity / cellular response to L-leucine / RHOBTB3 ATPase cycle / cullin-RING ubiquitin ligase complex / embryonic cleavage / cell projection organization / positive regulation of mitotic metaphase/anaphase transition / Cul7-RING ubiquitin ligase complex / ubiquitin-dependent protein catabolic process via the C-end degron rule pathway / Loss of Function of FBXW7 in Cancer and NOTCH1 Signaling / positive regulation of protein autoubiquitination / protein neddylation / Notch binding / NEDD8 ligase activity / RHOBTB1 GTPase cycle / Cul5-RING ubiquitin ligase complex / negative regulation of response to oxidative stress / ubiquitin-ubiquitin ligase activity / fibroblast apoptotic process / Cul4A-RING E3 ubiquitin ligase complex / SCF ubiquitin ligase complex / negative regulation of Rho protein signal transduction / Cul2-RING ubiquitin ligase complex / negative regulation of type I interferon production / Cul4B-RING E3 ubiquitin ligase complex / ubiquitin ligase complex scaffold activity / mitotic metaphase chromosome alignment / SCF-dependent proteasomal ubiquitin-dependent protein catabolic process / Cul3-RING ubiquitin ligase complex / intercellular bridge / mitotic spindle assembly checkpoint signaling / stress fiber assembly / Prolactin receptor signaling / positive regulation of cytokinesis / protein monoubiquitination / mitotic sister chromatid segregation / cullin family protein binding / ubiquitin-like ligase-substrate adaptor activity / sperm flagellum / protein autoubiquitination / RHOBTB2 GTPase cycle / protein K48-linked ubiquitination / Nuclear events stimulated by ALK signaling in cancer / endoplasmic reticulum to Golgi vesicle-mediated transport / gastrulation / 14-3-3 protein binding / positive regulation of TORC1 signaling / T cell activation / Regulation of BACH1 activity / negative regulation of autophagy / intrinsic apoptotic signaling pathway / cyclin binding / post-translational protein modification / positive regulation of protein ubiquitination / integrin-mediated signaling pathway / Degradation of DVL / Recognition of DNA damage by PCNA-containing replication complex / Degradation of GLI1 by the proteasome / Negative regulation of NOTCH4 signaling / cellular response to amino acid stimulus / GSK3B and BTRC:CUL1-mediated-degradation of NFE2L2 / Vif-mediated degradation of APOBEC3G / Hedgehog 'on' state / DNA Damage Recognition in GG-NER / Degradation of GLI2 by the proteasome / GLI3 is processed to GLI3R by the proteasome / FBXL7 down-regulates AURKA during mitotic entry and in early mitosis / protein destabilization / RING-type E3 ubiquitin transferase / Degradation of beta-catenin by the destruction complex / negative regulation of canonical Wnt signaling pathway / Oxygen-dependent proline hydroxylation of Hypoxia-inducible Factor Alpha / Dual Incision in GG-NER / Evasion by RSV of host interferon responses / NOTCH1 Intracellular Domain Regulates Transcription / Transcription-Coupled Nucleotide Excision Repair (TC-NER) / Formation of TC-NER Pre-Incision Complex / mitotic spindle / Wnt signaling pathway / Regulation of expression of SLITs and ROBOs / Constitutive Signaling by NOTCH1 PEST Domain Mutants / Constitutive Signaling by NOTCH1 HD+PEST Domain Mutants / Formation of Incision Complex in GG-NER / spindle pole / Interleukin-1 signaling / Orc1 removal from chromatin / Dual incision in TC-NER / G1/S transition of mitotic cell cycle Similarity search - Function | |||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.2 Å | |||||||||||||||
 Authors Authors | Wang W / Ling L / Dai Z / Zuo P / Yin Y | |||||||||||||||
| Funding support |  China, 4 items China, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: A conserved N-terminal motif of CUL3 contributes to assembly and E3 ligase activity of CRL3. Authors: Weize Wang / Ling Liang / Zonglin Dai / Peng Zuo / Shang Yu / Yishuo Lu / Dian Ding / Hongyi Chen / Hui Shan / Yan Jin / Youdong Mao / Yuxin Yin /  Abstract: The CUL3-RING E3 ubiquitin ligases (CRL3s) play an essential role in response to extracellular nutrition and stress stimuli. The ubiquitin ligase function of CRL3s is activated through dimerization. ...The CUL3-RING E3 ubiquitin ligases (CRL3s) play an essential role in response to extracellular nutrition and stress stimuli. The ubiquitin ligase function of CRL3s is activated through dimerization. However, how and why such a dimeric assembly is required for its ligase activity remains elusive. Here, we report the cryo-EM structure of the dimeric CRL3 complex and reveal a conserved N-terminal motif in CUL3 that contributes to the dimerization assembly and the E3 ligase activity of CRL3. We show that deletion of the CUL3 N-terminal motif impairs dimeric assembly and the E3 ligase activity of both CRL3 and several other CRL3s. In addition, we found that the dynamics of dimeric assembly of CRL3 generates a variable ubiquitination zone, potentially facilitating substrate recognition and ubiquitination. These findings demonstrate that a CUL3 N-terminal motif participates in the assembly process and provide insights into the assembly and activation of CRL3s. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36987.map.gz emd_36987.map.gz | 453.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36987-v30.xml emd-36987-v30.xml emd-36987.xml emd-36987.xml | 22 KB 22 KB | Display Display |  EMDB header EMDB header |
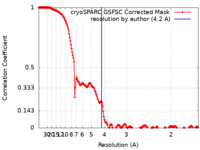
| FSC (resolution estimation) |  emd_36987_fsc.xml emd_36987_fsc.xml | 17 KB | Display |  FSC data file FSC data file |
| Images |  emd_36987.png emd_36987.png | 42.6 KB | ||
| Filedesc metadata |  emd-36987.cif.gz emd-36987.cif.gz | 6.4 KB | ||
| Others |  emd_36987_additional_1.map.gz emd_36987_additional_1.map.gz emd_36987_half_map_1.map.gz emd_36987_half_map_1.map.gz emd_36987_half_map_2.map.gz emd_36987_half_map_2.map.gz | 483.7 MB 474.8 MB 474.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36987 http://ftp.pdbj.org/pub/emdb/structures/EMD-36987 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36987 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36987 | HTTPS FTP |
-Validation report
| Summary document |  emd_36987_validation.pdf.gz emd_36987_validation.pdf.gz | 995 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_36987_full_validation.pdf.gz emd_36987_full_validation.pdf.gz | 994.6 KB | Display | |
| Data in XML |  emd_36987_validation.xml.gz emd_36987_validation.xml.gz | 26.1 KB | Display | |
| Data in CIF |  emd_36987_validation.cif.gz emd_36987_validation.cif.gz | 34.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36987 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36987 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36987 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36987 | HTTPS FTP |
-Related structure data
| Related structure data |  8k9iMC  8k8tC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_36987.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36987.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.821 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Raw map of CUL3(NA motif deletion)-RBX1-KLHL22 complex
| File | emd_36987_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Raw map of CUL3(NA motif deletion)-RBX1-KLHL22 complex | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map B
| File | emd_36987_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map A
| File | emd_36987_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : CUL3-RBX1-KLHL22 complex without CUL3 NA motif
| Entire | Name: CUL3-RBX1-KLHL22 complex without CUL3 NA motif |
|---|---|
| Components |
|
-Supramolecule #1: CUL3-RBX1-KLHL22 complex without CUL3 NA motif
| Supramolecule | Name: CUL3-RBX1-KLHL22 complex without CUL3 NA motif / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Cullin-3
| Macromolecule | Name: Cullin-3 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 87.432312 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MDEKYVNSIW DLLKNAIQEI QRKNNSGLSF EELYRNAYTM VLHKHGEKLY TGLREVVTEH LINKVREDVL NSLNNNFLQT LNQAWNDHQ TAMVMIRDIL MYMDRVYVQQ NNVENVYNLG LIIFRDQVVR YGCIRDHLRQ TLLDMIARER KGEVVDRGAI R NACQMLMI ...String: MDEKYVNSIW DLLKNAIQEI QRKNNSGLSF EELYRNAYTM VLHKHGEKLY TGLREVVTEH LINKVREDVL NSLNNNFLQT LNQAWNDHQ TAMVMIRDIL MYMDRVYVQQ NNVENVYNLG LIIFRDQVVR YGCIRDHLRQ TLLDMIARER KGEVVDRGAI R NACQMLMI LGLEGRSVYE EDFEAPFLEM SAEFFQMESQ KFLAENSASV YIKKVEARIN EEIERVMHCL DKSTEEPIVK VV ERELISK HMKTIVEMEN SGLVHMLKNG KTEDLGCMYK LFSRVPNGLK TMCECMSSYL REQGKALVSE EGEGKNPVDY IQG LLDLKS RFDRFLLESF NNDRLFKQTI AGDFEYFLNL NSRSPEYLSL FIDDKLKKGV KGLTEQEVET ILDKAMVLFR FMQE KDVFE RYYKQHLARR LLTNKSVSDD SEKNMISKLK TECGCQFTSK LEGMFRDMSI SNTTMDEFRQ HLQATGVSLG GVDLT VRVL TTGYWPTQSA TPKCNIPPAP RHAFEIFRRF YLAKHSGRQL TLQHHMGSAD LNATFYGPVK KEDGSEVGVG GAQVTG SNT RKHILQVSTF QMTILMLFNN REKYTFEEIQ QETDIPEREL VRALQSLACG KPTQRVLTKE PKSKEIENGH IFTVNDQ FT SKLHRVKIQT VAAKQGESDP ERKETRQKVD DDRKHEIEAA IVRIMKSRKK MQHNVLVAEV TQQLKARFLP SPVVIKKR I EGLIEREYLA RTPEDRKVYT YVAKLHHHHH H UniProtKB: Cullin-3 |
-Macromolecule #2: Kelch-like protein 22
| Macromolecule | Name: Kelch-like protein 22 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 20.36534 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAEEQEFTQL CKLPAQPSHP HCVNNTYRSA QHSQALLRGL LALRDSGILF DVVLVVEGRH IEAHRILLAA SCDYFRGMFA GGLKEMEQE EVLIHGVSYN AMCQILHFIY TSELELSLSN VQETLVAACQ LQIPEIIHFC CDFLMSWVDE ENILDVYRLA E LFDLSRLT EQLDTYILKN UniProtKB: Kelch-like protein 22 |
-Macromolecule #3: E3 ubiquitin-protein ligase RBX1, N-terminally processed
| Macromolecule | Name: E3 ubiquitin-protein ligase RBX1, N-terminally processed type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 12.289977 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAAAMDVDTP SGTNSGAGKK RFEVKKWNAV ALWAWDIVVD NCAICRNHIM DLCIECQANQ ASATSEECTV AWGVCNHAFH FHCISRWLK TRQVCPLDNR EWEFQKYGH UniProtKB: E3 ubiquitin-protein ligase RBX1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2.00 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| ||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Support film - Material: CARBON / Support film - topology: CONTINUOUS | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: DIFFRACTION / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

















 Z
Z Y
Y X
X