[English] 日本語
 Yorodumi
Yorodumi- EMDB-36709: Cryo-EM structure of bilirubin ditaurate (BDT) bound human ABC tr... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of bilirubin ditaurate (BDT) bound human ABC transporter ABCC2 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ATP-dependent transporter / conjugated organic anions transporter / ATP hydrolyzes / TRANSPORT PROTEIN / bilirubin / ABC transporter | |||||||||
| Function / homology |  Function and homology information Function and homology informationDefective ABCC2 causes DJS / bilirubin transmembrane transporter activity / bilirubin transport / xenobiotic export from cell / leukotriene transport / ABC-type glutathione-S-conjugate transporter / ABC-type glutathione S-conjugate transporter activity / ATPase-coupled inorganic anion transmembrane transporter activity / Atorvastatin ADME / heme catabolic process ...Defective ABCC2 causes DJS / bilirubin transmembrane transporter activity / bilirubin transport / xenobiotic export from cell / leukotriene transport / ABC-type glutathione-S-conjugate transporter / ABC-type glutathione S-conjugate transporter activity / ATPase-coupled inorganic anion transmembrane transporter activity / Atorvastatin ADME / heme catabolic process / organic anion transmembrane transporter activity / xenobiotic transmembrane transport / xenobiotic transport across blood-brain barrier / transepithelial transport / intercellular canaliculus / ABC-type xenobiotic transporter / Paracetamol ADME / ABC-type xenobiotic transporter activity / Translocases; Catalysing the translocation of other compounds; Linked to the hydrolysis of a nucleoside triphosphate / bile acid and bile salt transport / Heme degradation / Aspirin ADME / xenobiotic transmembrane transporter activity / ATPase-coupled transmembrane transporter activity / transport across blood-brain barrier / ABC-type transporter activity / xenobiotic metabolic process / ABC-family proteins mediated transport / transmembrane transport / apical plasma membrane / negative regulation of gene expression / cell surface / ATP hydrolysis activity / ATP binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.32 Å | |||||||||
 Authors Authors | Mao YX / Chen ZP / Wang L / Hou WT / Chen YX / Zhou CZ | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Transport mechanism of human bilirubin transporter ABCC2 tuned by the inter-module regulatory domain. Authors: Yao-Xu Mao / Zhi-Peng Chen / Liang Wang / Jie Wang / Cong-Zhao Zhou / Wen-Tao Hou / Yuxing Chen /  Abstract: Bilirubin is mainly generated from the breakdown of heme when red blood cells reach the end of their lifespan. Accumulation of bilirubin in human body usually leads to various disorders, including ...Bilirubin is mainly generated from the breakdown of heme when red blood cells reach the end of their lifespan. Accumulation of bilirubin in human body usually leads to various disorders, including jaundice and liver disease. Bilirubin is conjugated in hepatocytes and excreted to bile duct via the ATP-binding cassette transporter ABCC2, dysfunction of which would lead to Dubin-Johnson syndrome. Here we determine the structures of ABCC2 in the apo, substrate-bound and ATP/ADP-bound forms using the cryo-electron microscopy, exhibiting a full transporter with a regulatory (R) domain inserted between the two half modules. Combined with substrate-stimulated ATPase and transport activity assays, structural analysis enables us to figure out transport cycle of ABCC2 with the R domain adopting various conformations. At the rest state, the R domain binding to the translocation cavity functions as an affinity filter that allows the substrates of high affinity to be transported in priority. Upon substrate binding, the R domain is expelled from the cavity and docks to the lateral of transmembrane domain following ATP hydrolysis. Our findings provide structural insights into a transport mechanism of ABC transporters finely tuned by the R domain. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36709.map.gz emd_36709.map.gz | 31.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36709-v30.xml emd-36709-v30.xml emd-36709.xml emd-36709.xml | 15.6 KB 15.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_36709.png emd_36709.png | 28.6 KB | ||
| Filedesc metadata |  emd-36709.cif.gz emd-36709.cif.gz | 6.5 KB | ||
| Others |  emd_36709_half_map_1.map.gz emd_36709_half_map_1.map.gz emd_36709_half_map_2.map.gz emd_36709_half_map_2.map.gz | 59.3 MB 59.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36709 http://ftp.pdbj.org/pub/emdb/structures/EMD-36709 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36709 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36709 | HTTPS FTP |
-Validation report
| Summary document |  emd_36709_validation.pdf.gz emd_36709_validation.pdf.gz | 714.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_36709_full_validation.pdf.gz emd_36709_full_validation.pdf.gz | 713.8 KB | Display | |
| Data in XML |  emd_36709_validation.xml.gz emd_36709_validation.xml.gz | 12.4 KB | Display | |
| Data in CIF |  emd_36709_validation.cif.gz emd_36709_validation.cif.gz | 14.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36709 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36709 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36709 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36709 | HTTPS FTP |
-Related structure data
| Related structure data |  8jxqMC  8jx7C  8jxuC  8jy4C  8jy5C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_36709.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36709.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||
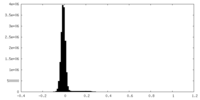
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_36709_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
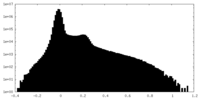
| Density Histograms |
-Half map: #1
| File | emd_36709_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
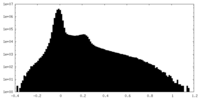
| Density Histograms |
- Sample components
Sample components
-Entire : ATP-binding cassette sub-family C member 2
| Entire | Name: ATP-binding cassette sub-family C member 2 |
|---|---|
| Components |
|
-Supramolecule #1: ATP-binding cassette sub-family C member 2
| Supramolecule | Name: ATP-binding cassette sub-family C member 2 / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: ATP-binding cassette sub-family C member 2
| Macromolecule | Name: ATP-binding cassette sub-family C member 2 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO EC number: Translocases; Catalysing the translocation of other compounds; Linked to the hydrolysis of a nucleoside triphosphate |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 176.964969 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MLEKFCNSTF WNSSFLDSPE ADLPLCFEQT VLVWIPLGYL WLLAPWQLLH VYKSRTKRSS TTKLYLAKQV FVGFLLILAA IELALVLTE DSGQATVPAV RYTNPSLYLG TWLLVLLIQY SRQWCVQKNS WFLSLFWILS ILCGTFQFQT LIRTLLQGDN S NLAYSCLF ...String: MLEKFCNSTF WNSSFLDSPE ADLPLCFEQT VLVWIPLGYL WLLAPWQLLH VYKSRTKRSS TTKLYLAKQV FVGFLLILAA IELALVLTE DSGQATVPAV RYTNPSLYLG TWLLVLLIQY SRQWCVQKNS WFLSLFWILS ILCGTFQFQT LIRTLLQGDN S NLAYSCLF FISYGFQILI LIFSAFSENN ESSNNPSSIA SFLSSITYSW YDSIILKGYK RPLTLEDVWE VDEEMKTKTL VS KFETHMK RELQKARRAL QRRQEKSSQQ NSGARLPGLN KNQSQSQDAL VLEDVEKKKK KSGTKKDVPK SWLMKALFKT FYM VLLKSF LLKLVNDIFT FVSPQLLKLL ISFASDRDTY LWIGYLCAIL LFTAALIQSF CLQCYFQLCF KLGVKVRTAI MASV YKKAL TLSNLARKEY TVGETVNLMS VDAQKLMDVT NFMHMLWSSV LQIVLSIFFL WRELGPSVLA GVGVMVLVIP INAIL STKS KTIQVKNMKN KDKRLKIMNE ILSGIKILKY FAWEPSFRDQ VQNLRKKELK NLLAFSQLQC VVIFVFQLTP VLVSVV TFS VYVLVDSNNI LDAQKAFTSI TLFNILRFPL SMLPMMISSM LQASVSTERL EKYLGGDDLD TSAIRHDCNF DKAMQFS EA SFTWEHDSEA TVRDVNLDIM AGQLVAVIGP VGSGKSSLIS AMLGEMENVH GHITIKGTTA YVPQQSWIQN GTIKDNIL F GTEFNEKRYQ QVLEACALLP DLEMLPGGDL AEIGEKGINL SGGQKQRISL ARATYQNLDI YLLDDPLSAV DAHVGKHIF NKVLGPNGLL KGKTRLLVTH SMHFLPQVDE IVVLGNGTIV EKGSYSALLA KKGEFAKNLK TFLRHTGPEE EATVHDGSEE EDDDYGLIS SVEEIPEDAA SITMRRENSF RRTLSRSSRS NGRHLKSLRN SLKTRNVNSL KEDEELVKGQ KLIKKEFIET G KVKFSIYL EYLQAIGLFS IFFIILAFVM NSVAFIGSNL WLSAWTSDSK IFNSTDYPAS QRDMRVGVYG ALGLAQGIFV FI AHFWSAF GFVHASNILH KQLLNNILRA PMRFFDTTPT GRIVNRFAGD ISTVDDTLPQ SLRSWITCFL GIISTLVMIC MAT PVFTII VIPLGIIYVS VQMFYVSTSR QLRRLDSVTR SPIYSHFSET VSGLPVIRAF EHQQRFLKHN EVRIDTNQKC VFSW ITSNR WLAIRLELVG NLTVFFSALM MVIYRDTLSG DTVGFVLSNA LNITQTLNWL VRMTSEIETN IVAVERITEY TKVEN EAPW VTDKRPPPDW PSKGKIQFNN YQVRYRPELD LVLRGITCDI GSMEKIGVVG RTGAGKSSLT NCLFRILEAA GGQIII DGV DIASIGLHDL REKLTIIPQD PILFSGSLRM NLDPFNNYSD EEIWKALELA HLKSFVASLQ LGLSHEVTEA GGNLSIG QR QLLCLGRALL RKSKILVLDE ATAAVDLETD NLIQTTIQNE FAHCTVITIA HRLHTIMDSD KVMVLDNGKI IECGSPEE L LQIPGPFYFM AKEAGIENVN STKFLEDYKD DDDKVEHHHH HHHH UniProtKB: ATP-binding cassette sub-family C member 2 |
-Macromolecule #2: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 2 / Number of copies: 1 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Macromolecule #3: 2-[3-[5-[(E)-(4-ethenyl-3-methyl-5-oxidanylidene-pyrrol-2-ylidene...
| Macromolecule | Name: 2-[3-[5-[(E)-(4-ethenyl-3-methyl-5-oxidanylidene-pyrrol-2-ylidene)methyl]-2-[[5-[(3-ethenyl-4-methyl-5-oxidanylidene-pyrrol-2-ylidene)methyl]-4-methyl-3-[3-oxidanylidene-3-(2-sulfoethylamino) ...Name: 2-[3-[5-[(E)-(4-ethenyl-3-methyl-5-oxidanylidene-pyrrol-2-ylidene)methyl]-2-[[5-[(3-ethenyl-4-methyl-5-oxidanylidene-pyrrol-2-ylidene)methyl]-4-methyl-3-[3-oxidanylidene-3-(2-sulfoethylamino)propyl]-1H-pyrrol-2-yl]methyl]-4-methyl-1H-pyrrol-3-yl]propanoylamino]ethanesulfonic acid type: ligand / ID: 3 / Number of copies: 1 / Formula: FEI |
|---|---|
| Molecular weight | Theoretical: 798.925 Da |
| Chemical component information |  ChemComp-FEI: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 55.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.32 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 368404 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)