+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
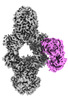
| Title | Structure of Gabija GajA-GajB 4:1 complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Anti-phage / IMMUNE SYSTEM | |||||||||
| Function / homology |  Function and homology information Function and homology informationrecombinational repair / 3'-5' DNA helicase activity / defense response to virus / endonuclease activity / Hydrolases; Acting on ester bonds / hydrolase activity / DNA binding / ATP binding / metal ion binding Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.39 Å | |||||||||
 Authors Authors | Li J / Wang Z / Wang L | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2024 Journal: Nature / Year: 2024Title: Structures and activation mechanism of the Gabija anti-phage system. Authors: Jing Li / Rui Cheng / Zhiming Wang / Wuliu Yuan / Jun Xiao / Xinyuan Zhao / Xinran Du / Shiyu Xia / Lianrong Wang / Bin Zhu / Longfei Wang /   Abstract: Prokaryotes have evolved intricate innate immune systems against phage infection. Gabija is a highly widespread prokaryotic defence system that consists of two components, GajA and GajB. GajA ...Prokaryotes have evolved intricate innate immune systems against phage infection. Gabija is a highly widespread prokaryotic defence system that consists of two components, GajA and GajB. GajA functions as a DNA endonuclease that is inactive in the presence of ATP. Here, to explore how the Gabija system is activated for anti-phage defence, we report its cryo-electron microscopy structures in five states, including apo GajA, GajA in complex with DNA, GajA bound by ATP, apo GajA-GajB, and GajA-GajB in complex with ATP and Mg. GajA is a rhombus-shaped tetramer with its ATPase domain clustered at the centre and the topoisomerase-primase (Toprim) domain located peripherally. ATP binding at the ATPase domain stabilizes the insertion region within the ATPase domain, keeping the Toprim domain in a closed state. Upon ATP depletion by phages, the Toprim domain opens to bind and cleave the DNA substrate. GajB, which docks on GajA, is activated by the cleaved DNA, ultimately leading to prokaryotic cell death. Our study presents a mechanistic landscape of Gabija activation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36569.map.gz emd_36569.map.gz | 197.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36569-v30.xml emd-36569-v30.xml emd-36569.xml emd-36569.xml | 15.5 KB 15.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_36569.png emd_36569.png | 93 KB | ||
| Filedesc metadata |  emd-36569.cif.gz emd-36569.cif.gz | 5.8 KB | ||
| Others |  emd_36569_half_map_1.map.gz emd_36569_half_map_1.map.gz emd_36569_half_map_2.map.gz emd_36569_half_map_2.map.gz | 194.3 MB 194.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36569 http://ftp.pdbj.org/pub/emdb/structures/EMD-36569 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36569 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36569 | HTTPS FTP |
-Validation report
| Summary document |  emd_36569_validation.pdf.gz emd_36569_validation.pdf.gz | 1.3 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_36569_full_validation.pdf.gz emd_36569_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  emd_36569_validation.xml.gz emd_36569_validation.xml.gz | 15.5 KB | Display | |
| Data in CIF |  emd_36569_validation.cif.gz emd_36569_validation.cif.gz | 18.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36569 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36569 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36569 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36569 | HTTPS FTP |
-Related structure data
| Related structure data |  8jqcMC  8jq9C  8jqbC  8wy4C  8wy5C  8x51C  8x5iC  8x5nC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_36569.map.gz / Format: CCP4 / Size: 209.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36569.map.gz / Format: CCP4 / Size: 209.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.84 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_36569_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
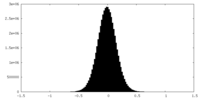
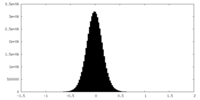
| Density Histograms |
-Half map: #1
| File | emd_36569_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
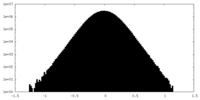
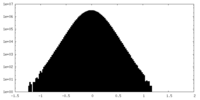
| Density Histograms |
- Sample components
Sample components
-Entire : 4:1 GajAB complex
| Entire | Name: 4:1 GajAB complex |
|---|---|
| Components |
|
-Supramolecule #1: 4:1 GajAB complex
| Supramolecule | Name: 4:1 GajAB complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 335 KDa |
-Macromolecule #1: Endonuclease GajA
| Macromolecule | Name: Endonuclease GajA / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO / EC number: Hydrolases; Acting on ester bonds |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 67.079469 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKFSNITIKN FRNFEKVNIN LDNKNVIFGM NDIGKTNFLY ALRFLLDKEI RKFGFNKSDY HKHDTSKKIE IILTLDLSNY EKDEDTKKL ISVVKGARTS ANADVFYIAL ESKYDDKELY GNIILKWGSE LDNLIDIPGR GNINALDNVF KVIYINPLVD L DKLFAQNK ...String: MKFSNITIKN FRNFEKVNIN LDNKNVIFGM NDIGKTNFLY ALRFLLDKEI RKFGFNKSDY HKHDTSKKIE IILTLDLSNY EKDEDTKKL ISVVKGARTS ANADVFYIAL ESKYDDKELY GNIILKWGSE LDNLIDIPGR GNINALDNVF KVIYINPLVD L DKLFAQNK KYIFEESQGN ESDEGILNNI KSLTDQVNQQ IGEMTIIKGF QQEITSEYRS LKKEEVSIEL KSEMAIKGFF SD IIPYIKK DGDSNYYPTS GDGRRKMLSY SIYNYLAKKK YEDKIVIYLI EEPEISLHRS MQIALSKQLF EQSTYKYFFL STH SPELLY EMDNTRLIRV HSTEKVVCSS HMYNVEEAYG SVKKKLNKAL SSALFAERVL LIEGPSEKIL FEKVLDEVEP EYEL NGGFL LEVGGTYFNH YVCTLNDLGI THIIKTDNDL KSKKGKKGVY ELLGLNRCLN LLGRENLDEI TIDIPEDIKG KKKKE RLNE RKKEIFKQYK NEVGEFLGER IYLSEIDLEN DLYSAIGESM KRIFENEDPV HYLQKSKLFN MVELVNNLST KDCFDV FEH EKFACLKELV GSDRG UniProtKB: Endonuclease GajA |
-Macromolecule #2: Gabija protein GajB
| Macromolecule | Name: Gabija protein GajB / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 57.757656 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MIEDEMSREQ IIKDGGNILV TAGAGSGKTT ILVSKIEADL KENKTHYSIA AVTFTNKAAK EIEGRLGYSS RGNFIGTNDG FVESEIIRP FIKDAFGNDY PDNFTAEYFD NQFASYDKGL QVLKYQNILG TYSNPKKNFK FQLALDILKK SLVARQYIFS K YFKIFIDE ...String: MIEDEMSREQ IIKDGGNILV TAGAGSGKTT ILVSKIEADL KENKTHYSIA AVTFTNKAAK EIEGRLGYSS RGNFIGTNDG FVESEIIRP FIKDAFGNDY PDNFTAEYFD NQFASYDKGL QVLKYQNILG TYSNPKKNFK FQLALDILKK SLVARQYIFS K YFKIFIDE YQDSDKDMHN LFMYLKDQLK IKLFIVGDPK QSIYIWRGAE PENFNGLIEN STDFNKYHLT SNFRCCQDIQ NY SNLFNEE TRSLIKEKNE VQNVISIADD MPISDILLKL TEEKQVLNIE AELVILVRRR NQAIEIMKEL NEEGFNFIFI PQT PLDRAT PNATLLKEVI KYVKNDRYSI YDLAAEIVGN LSSREIKEIQ KIINELLVPN INQVLINQVL INLFAKLEIT LDTR EITAF TEVMMTNEFD IAFDTNEYLH KIFTVHSAKG LEFNQVIITA SDYNVHYNRD TNEHYVATTR AKDKLIVIMD NKKYS DYIE TLMKELKIKN IIKSI UniProtKB: Gabija protein GajB |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 1.6 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: INSILICO MODEL |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.39 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 4218276 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-8jqc: |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)